Environmental Engineering Reference
In-Depth Information
(provided by a filter), is preferred as its spectral emission distribution is very close to the
solar radiation spectrum (Marcheterre et al. 1988). It has been demonstrated that the use
of different light sources under identical aqueous conditions can produce similar degrada-
tion products, with the only difference being in their kinetics of formation (Barceló et al.
1996; Marcheterre et al. 1988). As for the experimental equipment, quartz glass is preferred
instead of other glass material since it permits a greater transmission of radiation (Peñuela
et al. 2000).
The photodegradation of organophosphorus, phenylurea, and cyclohexanedione oxime
pesticides has been studied in a variety of different aquatic media. In many cases, signifi-
cant differences in the degradation rates were obtained, suggesting a strong dependence
on the type of the aquatic media (Durand and Barceló 1990; Durand et al. 1990).
Sevilla-Morán and coworkers (2010b) investigated the photodegradation of the her-
bicide sethoxydim-lithium in ultrapure and natural waters (mineral, well, and river).
Half-lives of the herbicides were higher in natural waters than in ultrapure waters, with
the photolysis rate decreasing in the following order: river < well ~ mineral < ultrapure
water. Sethoxydim-lithium almost totally degraded after 5 h in ultrapure water versus
10 h in river water. The composition of aquatic media also plays an important role in
the phototransformation of pesticides. Various authors point out that particulate mat-
ter, such as sediment particles, and dissolved substances present in natural waters could
be responsible for the different photolysis rates observed between natural and distilled
water (Dimou et al. 2004a; Schwarzenbach Rene et al. 2002; Tchaikovskaya et al. 2007). The
most important light-absorbing species that may induce indirect photolytic transforma-
tion of organic pollutants in natural waters are the chromophores present in DOM, where
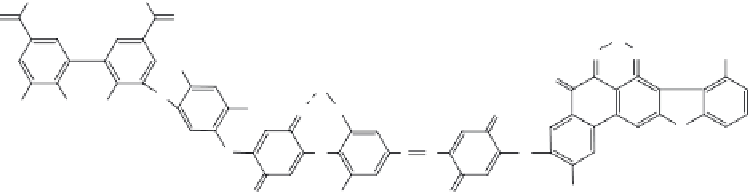
HAs (Figure 4.2) and, to a lesser extent, fulvic acids (FAs) are the important absorbing
constituents. The complex structure of HA is basically composed of phenolic, carbonylic,
and carboxylic acids, and they absorb radiation in the range of 300-600 nm (Sevilla Morán
2010). Their concentration in natural waters ranges from less than 5 ml/L in groundwater
and sea water to 25 mg/L in some rivers (Barceló and Hennion 1997; Dimou et al. 2005).
Diverse studies are available from the literature, in which HA act by enhancing (Sakkas
et al. 2002a,b; Santoro et al. 2000; Vialaton and Richard 2002) or inhibiting (Bachman and
Patterson 1999; Dimou et al. 2004a, 2005; Elazzouzi et al. 1999; Sevilla-Morán et al. 2008,
2010a) the degradation of pesticides. In the first case, HA behave as a “sensitizer,” in which
the excited states of HA can participate in a charge-transfer interaction with pesticides
or generate reactive intermediates, such as hydroxyl radicals, singlet oxygen, solvated
electrons, or hydrogen peroxide. In the second case, HAs act as photon trap (optical filter
effect), decreasing the photodegradation rate of pesticides.
OH
HO
O
O
H
OH
O
O
HO
O
H
O
HO
OH HO
O
NH
2
O
O
O
O
O
C
N
O
OH
O
O
H
2
N
FIGURE 4.2
Chemical structure of humic acids.
Search WWH ::

Custom Search