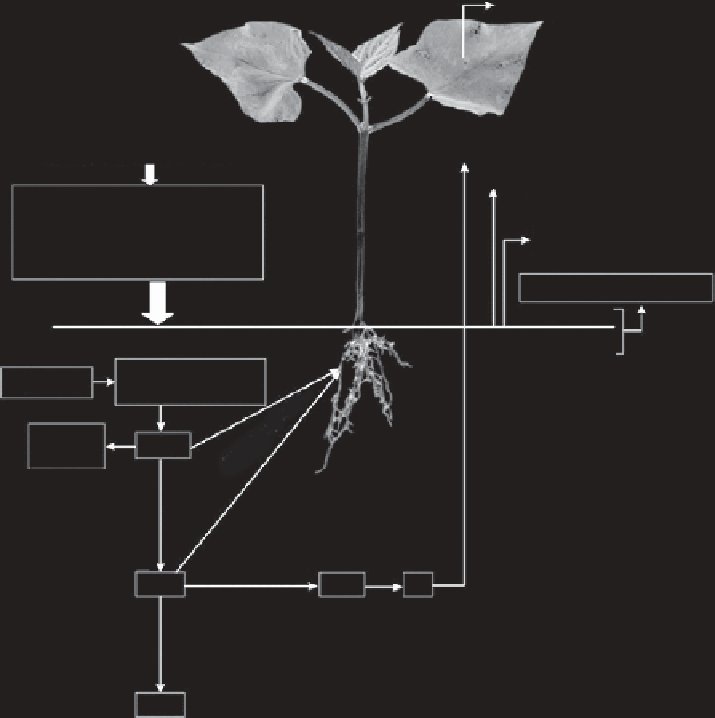
Agriculture Reference
In-Depth Information
NH
3
volatilization from
bean foliage
N
2
O + N
2
N addition to soil
NH
3
volatilization from
soil surface
• Fertilizers
• Biological and non-biological
• Oraganic manures
• Atmospheric N
2
Loss through
surface runoff
H
2
O oxidized zone
Mineralization
(ammonification)
Organic N
NH
+
fixation
NH
+
Denitrification
NO
3
-
N
2
O
N
2
NO
3
-
FIGURE 2.1
A simplified version of N cycle in soil-plant system. (From Fageria, N. K. 2009.
The Use of
Nutrients in Crop Plants
. Boca Raton, Florida: CRC Press. With permission.)
nutrients, including N and energy. Apart from abiotic factors such as soil temperature, soil water
content, and soil aeration, the properties of the organic amendments themselves affect the decom-
position process or mineralization (Nett et al., 2012).
According to Stevenson (1982), mineralization is the conversion of organic forms of N into NH
4
+
and
NO
3
−
. The initial conversion into
NH
4
+
is referred to as ammonification and the oxidation of
this compound to
NO
3
−
is termed nitrification. Nitrification is one of the key processes determining
the efficiency of fertilizer use by crops, as well as N losses from soil through leaching of
NO
2
−
and
NO
3
−
and emissions of N
2
O and N
2
gas resulting from denitrification and anaerobic
NH
4
+
oxidation,
respectively (Nieder and Benbi, 2008; Wu et al., 2011). The nitrification process occurs in two
phases in the soil-plant system and can be represented by the following equations:
2NH3O2NO 2H O H
2NOO 2NO
+
+ ⇔ +
-
+
+
4
2
2
2
−
+⇔
-
2
2
3
In the process of nitrification, bacteria known as
Nitrosomonas
are involved in the process of
conversion of ammonia into nitrites and the bacteria, which convert nitrites into nitrates, are known
as
Nitrobacter
. Collectively, the nitrifying organisms are known as
Nitrobacteria
. Under optimal