Environmental Engineering Reference
In-Depth Information
1.2.7.3 Vapor Pressure Differences
A stabilizer with a vapor pressure that is signii cantly lower than the host solvent will be partitioned
into the still bottoms. Stabilizers whose vapor pressures are signii cantly higher than the host solvent
will be progressively depleted as vapor is lost during operation of the degreaser or dry cleaner. Vapor
pressure differences at ambient temperatures may contribute to slow loss or enrichment of stabilizers
in cold cleaning operations where the solvent is used at room temperature. The literature usually has
experimental vapor pressure values available for chemicals at standard temperature and pressure,
that is, at 25°C and 1 atm pressure. The vapor pressures of substances change with temperature. The
relevant indicator of the potential for stabilizer partitioning is the vapor pressure of the stabilizer at
the boiling temperature of the solvent. Obtaining stabilizer vapor pressures at solvent boiling tem-
peratures is more difi cult as less experimental data are available.
Several methods are available for calculating vapor pressure at different temperatures, however.
The most common method uses the Antoine equation:
log
10
P
=
A
−
[
B
/(
T
+
C
)],
(1.23)
where
P
is the vapor pressure (in mm Hg),
T
is the temperature (in °C), and
A
,
B
, and
C
are the
Antoine constants for a particular chemical. The Antoine constants are available in
Yaws' Handbook
of Antoine Coefi cients for Vapor Pressure
(Knovel Corporation, 2006). The Antoine equation does
not apply to all compounds and only applies to a specii c temperature range for each compound.
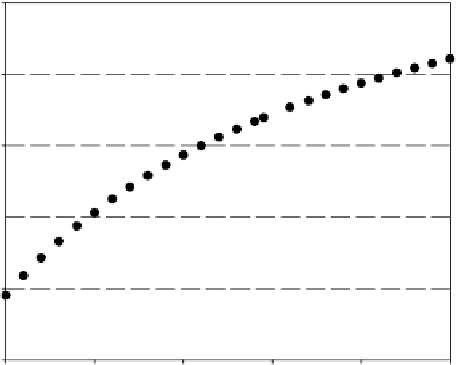
Figure 1.3 shows the vapor pressure of 1,4-dioxane and the vapor pressure of methyl chloroform; the
plot graphically presents the tendency of 1,4-dioxane to concentrate.
1.2.7.4 Carbon Adsorption
The use of activated charcoal i lters for on-site purii cation of solvents to remove oily waste can
contribute to the depletion of stabilizers (Dow Chemical Company, 2006b). Acid acceptors in
particular are prone to remain adsorbed to carbon i lters used to i lter spent solvents for reuse
(Howell and Tarrer, 1994). The afi nity of an organic compound to adsorb to carbon is commonly
taken as the ratio of the chemical adsorbed per unit weight of organic carbon, referred to as a
100,000
1,4-Dioxane
Methyl chloroform
10,000
1000
100
10
1
0
50
100
150
200
250
Temperature (°C)
FIGURE 1.3
Vapor pressures of 1,4-dioxane and methyl chloroform. [1,4-Dioxane data from Vinson, C.G.,
Jr. and Martin, J.J., 1963,
Journal of Chemical Engineering Data
8(1): 74-75. Methyl chloroform values cal-
culated by using Antoine equation and constants from Knovel Corporation, 2006.]



Search WWH ::

Custom Search