Environmental Engineering Reference
In-Depth Information
differences between the 1,4-dioxane concentration decrease from ozone
Pd
catalyst. Suh et al. noted that oxidation of 1,4-dioxane to more easily biodegraded intermediates
would support the integration of advanced oxidation and more conventional biological treatment
methods for wastewater.
+
peroxide or ozone
+
7. 7.9 S
ONOCHEMICAL
O
XIDATION
Sonochemical oxidation of organic contaminants is caused by the production of hydroxyl radicals
and other oxidizing species during the collapse of cavitation bubbles formed during high-frequency
acoustic stimulation of the aqueous media. High temperatures (up to 4000°C) and pressures (1000
atmospheres) can be present in the cavitation bubbles when they collapse. VOCs are degraded
through combustion, high-temperature chemical reactions, supercritical water oxidation, and the
production of free radicals. Hydroxyl and other free radicals formed during this process are capable
of oxidizing organic compounds, such as 1,4-dioxane.
Beckett and Hua (2000) and Hua (2000) performed an evaluation of the viability of using sono-
chemical destruction for 1,4-dioxane and dei ned the decomposition products and pathways for
destruction of 1,4-dioxane during sonolysis at discrete ultrasonic frequencies. Ninety-six percent of
the 1,4-dioxane was completely broken down in the i rst 120 min, and Beckett and Hua (2000) and
Hua (2000) identii ed i ve major reaction intermediates: ethylene glycol diformate (EGD), meth-
oxyacetic acid, formic acid, glycolic acid, and formaldehyde (Figure 7.15). In general, these break-
down products are less toxic and less recalcitrant than 1,4-dioxane. The frequency proved to be an
important factor because the bubble size, and resultant collapse energy, is inversely correlated to the
sonic frequency.
Beckett and Hua (2003) assessed how the addition of ferrous iron affected 1,4-dioxane sono-
chemical degradation rates. Ferrous iron addition increased the degradation rate and efi ciency of
1,4-dioxane decomposition in all tested ultrasonic frequencies. The combination of ferrous iron and
ultrasound yielded a higher concentration of the hydroxyl radical, thereby causing more rapid
destruction than either sonication or Fenton's reagent alone. Nakajima et al. (2007) further assessed
the effect on sonochemical degradation rates by addition of reduced titanium dioxide powder, as
1,4-dioxane
Ethylene glycol diformate
Formaldehyde
Glycolic acid
Formic acid
Methoxyacetic acid
1
0.8
0.6
0.4
0.2
0
0
20
40
60
80
100
120
Time (min)
FIGURE 7.15
Formation of sonolytic by-products of 1,4-dioxane over time. (From Beckett, M.A. and Hua,
I., 2000,
Environmental Science and Technology
34(19): 3944-3953.)






















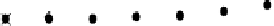















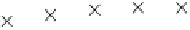
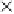


















Search WWH ::

Custom Search