Environmental Engineering Reference
In-Depth Information
Schreier et al. (2006) performed a series of bench-scale studies assessing the viability of using
ozone alone for ISCO, as opposed to mixing ozone and hydrogen peroxide
in situ
, which can be
difi cult in heterogeneous aquifer materials. The work was performed in support of remedial action
at the Cooper Drum Superfund site in South Gate, Los Angeles County, California, where 1,4-dioxane
concentrations targeted for treatment by ISCO were as high as 750
g/L (Yunker, 2007). Peroxone,
the mixture of ozone and hydrogen peroxide, is a demonstrated oxidizer, as already described, but
ozone alone had been commonly regarded as not being of sufi cient oxidation potential to destroy
1,4-dioxane. Ozone sparging of site groundwater resulted in the complete removal of 1,4-dioxane.
To assess whether the removal was due to volatilization or destruction, vapor samples were col-
lected from the off-gas and a sparge with inert gas (nitrogen) was evaluated. The data indicate that
1,4-dioxane removal was due to oxidation. Schreier et al. postulated that naturally occurring inor-
ganic materials in the site soil or groundwater were enhancing the capability of ozone, which could
eliminate or reduce the need for hydrogen peroxide. Ferrous iron, chelated iron, bicarbonate, and
cocontaminants such as TCE were evaluated as potential oxidation enhancers. A test of spiked
deionized water indicated that ozone alone was capable of reducing concentrations from 400 to
98
μ
g/L. Additional tests run with ferrous iron, chelated
iron, and bicarbonate resulted in reductions down to the detection limit. 1,4-Dioxane removal in the
presence of TCE did not signii cantly differ from the removal observed with ozone alone. Schreier
et al. (2006) concluded that natural waters may contain elements or compounds that allow ozone to
succeed at complete 1,4-dioxane removal.
A follow-on i eld pilot study at the same site (Sadeghi et al., 2006; Sadeghi and Gruber, 2007;
USEPA, 2007) involved the use of specially constructed wells with an ozone diffuser in the bottom
of the well and a hydrogen peroxide diffuser higher in the well, such that the two amendments inter-
act in the subsurface to form the hydroxyl radical. This system is patented by Applied Process
Te ch nolog y a s t he P u lse - OX
TM
(Applied Process Technology, Inc., Pleasant Hill, CA) system. Initial
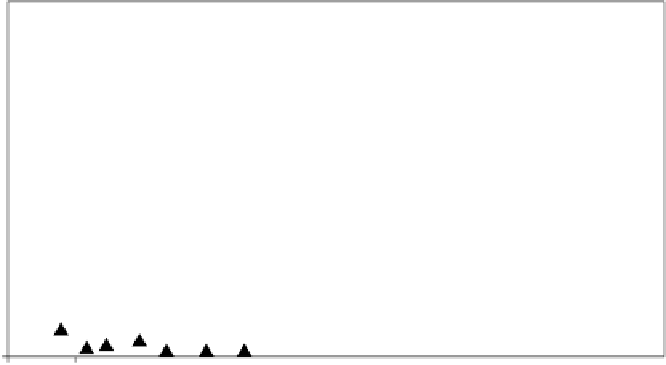
injections were of ozone alone, which resulted in signii cant reductions in 1,4-dioxane (and TCE)
concentrations (Figure 7.12). Five months later, hydrogen peroxide injections were initiated, and it
was determined that optimal results were achieved with the combination of 16% hydrogen peroxide
and 2 pounds per day per injection well or 1 pound per day per injection interval of ozone. The
μ
g/L, but not to the detection limit of 3
μ
1000
TCE
cis
900
-1, 2-DCE
1,1-DCA
1,4-Dioxane
800
O
3
700
increased
Focused
in
je
ction
600
End of
pilot test
500
400
300
200
100
0
-50
0
50
100
150
200
Days
250
300
350
400
450
FIGURE 7.12
ISCO i eld pilot study results. (From Sadeghi, V.M. and Gruber, D.J., 2007,
In situ
oxidation
of 1,4-dioxane with ozone and hydrogen peroxide. Poster presentation at URS's Environmental Technology
and Management Seminar, Oakland, CA.)





































Search WWH ::

Custom Search