Environmental Engineering Reference
In-Depth Information
et al., 2006). The same study found that increases in concentrations of target ether and alcohol
analytes in headspace above a liquid sample at higher temperatures are partially offset by a lower
sorbent-air partition constant at higher temperatures. Because sorption is an exothermic process,
the sorbent-air partition coefi cient decreases as the sorbent temperature in the SPDE needle
increases. The maximum extraction yields therefore occur at intermediate temperatures, with a
decrease in SPDE extraction yield at higher temperatures (Jochmann et al., 2006). For SPDE, it is
also important that the selected temperature not be so cool as to allow the condensation of water
vapor in the needle, to prevent the 1,4-dioxane peak deterioration with increasing water content
already noted (Kawata et al., 2001; Jochmann et al., 2006). The duration of extraction by SPME and
the number of cycles of extraction by SPDE also have a signii cant bearing on the extraction recov-
ery of 1,4-dioxane from water samples. Recovery of 1,4-dioxane approximately doubled when the
number of SPDE extraction cycles was increased from 10 to 30; no appreciable change was observed
when the number of SPDE extraction cycles was increased from 30 to 50 (Jochmann et al., 2006).
One of the lowest MDLs reported in the peer-reviewed literature for analysis of 1,4-dioxane in
water, 130 parts per trillion, was achieved with SPE with solvent desorption (Isaacson et al., 2006).
The method was developed at Oregon State University (OSU) and involved the following steps:
25-mm-diameter activated carbon disks with 10 μm particles and 1100 m
2
/g surface area were i tted
to polypropylene disk holders and attached to a vacuum manifold. Sample volumes of 80 mL were
spiked with 100
g/mL dioxane-d
8
. Samples were pulled through dry disks under a 67 kPa
vacuum, followed by 1 h of drying in laboratory air under vacuum. Disks were then transferred into
2 mL GC autosampler vials, to which acetone and instrumental standards were added. Acetone was
found to yield greater than 90%, that is, the same recovery as dichloromethane. Acetone is preferred
over dichloromethane because it is less hazardous and wastes are more easily managed.
The parameters of GC-MS/MS analysis for the OSU SPE method are summarized in Appendix 3. The
pilot study for development of this method also tested the effectiveness of SPE for extraction and iden-
tii cation of two other stabilizer compounds, THF and 1,3-dioxolane. Spike and recovery experiments
with THF and 1,3-dioxolane in water yielded greater than 80% recovery for THF and less than 10% for
1,3-dioxolane. Breakthrough of the 1,3-dioxolane was coni rmed from the fact that 90% of the added
mass was recovered from the water that passed through the disk, as determined by an in-vial LLE
method. 1,3-Dioxolane is poorly suited for extraction by SPE using activated carbon. The MDL for 1,4-
dioxane was 0.13
μ
L of 50
μ
g/L and the LOQ
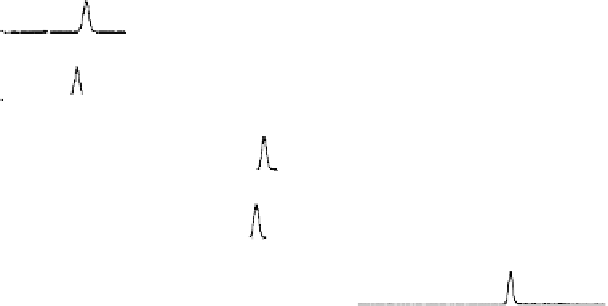
was 3.1 ppb (Isaacson et al., 2006). Chromatograms from the OSU study are shown in Figure 4.6.
μ
g/L, and the LOQ was 0.31
μ
g/L. For THF, the MDL was 1.0
μ
100
0
THF
m
/
z
73
55
100
0
THF-d
8
m
/
z
81
62
100
m
/
z
89
45
Dioxane
0
100
Dioxane-d
8
m
/
z
97
49
0
m
z
/
117
61
100
0
Butyl acetate
4.50
5.00
5.50
6.00
6.50
7.00
7.50
8.00
8.50
9.00
9.50
10.00
Time (min)
FIGURE 4.6
Chromatograms of THF, THF-d
8
, dioxane, dioxane-d
8
, and internal standard butyl acetate. [From
Isaacson, C., Mohr, T.K.G., and Field, J.A., 2006,
Environmental Science & Technology
40(23): 7305-7311;
Chromatograms by Oregon State University. With permission.]






Search WWH ::

Custom Search