Environmental Engineering Reference
In-Depth Information
(a)
(b)
(c)
NO
OO
O
O
O
O
OH/O
2
O
O
O
NO
2
1,4-dioxane
Rapid
(e)
(d)
H
O
H
O
CH
2
CH
2
OO
+ O
2
O
O
O
O
NO
NO
2
H
O
CH
2
O
(f )
O
O
O
2
HO
2
O
H
O
OH
Products
O
H
(g)
O
Ethylene glycol diformate
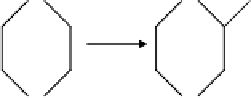
FIGURE 3.2
Photo-oxidation pathway for 1,4-dioxane in the presence of NO
x
. Note: the product of step (f),
ethylene glycol diformate, is also called ethylene-1,2-diformate (EDF). (From Geiger, H., Maurer, T., and Becker,
K.H., 1999,
Chemical Physics Letters
314: 465-471. With permission.)
Although the OH
•
concentration in the atmosphere varies with location, time of day, season,
and meteorological conditions, a reasonable 24-hour global average atmospheric lifetime for
1,4- dioxane is 1-2 days (Platz et al., 1997). 1,3-Dioxolane follows a similar hydroxyl radical
oxidation pathway in which ethylene carbonate and methylene glycol diformate are produced
(Freitas-Dinis et al., 2001).
Atomic chlorine is another important atmospheric oxidant capable of oxidizing 1,4-dioxane.
Chlorine atoms are introduced into the atmosphere through reactions of HCl with OH and by
photolysis of chlorine molecules from industrial emissions and sea salt. The chlorine atoms play a










Search WWH ::

Custom Search