Environmental Engineering Reference
In-Depth Information
(h)
(i)
(f)
(g)
CH
3
H
H
H
C OH
OH
C
O
O
O
O
O
O
O
O
O
O
O
O
+
+
H
+
CH
3
O
O
O
O
O
O
O
O
O
O
O
O
h
n
(e)
CH
3
CHO
O
O
O
O
h
n
h
n
h
n
CH
2
CH
2
OCH
2
CHO
CH
3
CH
2
OCH
2
CHO+
O
O
O
O
O
O
(a)
(b)
(c)
(d)
O
O
FIGURE 3.1
Laboratory photolysis of 1,4-dioxane [A] produces a dioxyl radical [B] by abstracting a hydro-
gen from dioxane (indicated by the arrow and dot in [B]). A pair of dioxane dimers ([H] and [I]) is formed
by the dimerization of dioxyl radicals. The dioxyl radical also undergoes bond cleavage to open the ring and
form radical [C], which then forms ethoxyacetaldehyde [D] and, with further light exposure, acetaldehyde [E].
Acetaldehyde can be photo-reduced to the alcohol isomers [F] and [G].
Note
: These reactions are not expected
to occur in the natural environment. (From Mazzocchi, P.H., and Bowen, M.W., 1975,
Journal of Organic
Chemistry
40(18): 2689-2690. With permission.)
Some experiments have sought to replicate the light range to which 1,4-dioxane would be exposed
in the near-surface atmosphere. One experiment simulated UV light with a short-wavelength cutoff
of 290 nm and an intensity 2.6 times greater than the noonday sun found in Freeport, Texas (Dilling
et al., 1976). Half the mass of 1,4-dioxane disappeared in 3.4 h, giving a corrected half-life of 8.8 h
to account for the artii cially enhanced light intensity. However, this experiment was conducted in
the presence of 5 ppm nitrous oxide, an oxidant involved in photo-oxidation, as discussed further in
Section 3.1.4.2
.
3.1.4.1.2 Photolysis in Surface Water
The degree to which light penetrates surface water governs the degree to which chemicals in solution
are photolyzed. The rates of photolytic reactions in a water body are affected by the solar spectral
irradiance at the water surface, radiative transfer of light from air into water, and the transmission
of sunlight in the water body.
Indirect or “sensitized” photolysis occurs in surface water when light-absorbing natural organic
compounds absorb photons. These compounds, called chromophores, can transfer energy in the form
of electrons or hydrogen ions to another compound. Titanium dioxide and other inorganic com-
pounds can also serve as an intermediate in the indirect photolysis of organic contaminants (Hemond
and Fechner, 1994). The degree to which contaminants in surface water may be susceptible to
photolysis or indirect photodegradation from chromophores is limited by the depth of light penetra-
tion in the surface-water body. Light penetration in water is governed by light intensity,
*
the angle of
light entry, turbidity, and dissolved organic matter in water. Light intensity at the water surface
decreases with the angle of sunlight entering the water; therefore, intensity decreases from midday to
sunset, from summer to winter, and from the tropics to higher latitudes, particularly in the UV-B
range (280-320 nm), but also for visible light (UV-A, 320-400 nm) (Zepp and Cline, 1977). Intensity
*
Light intensity is the number of photons per unit surface area per unit time.



























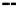
Search WWH ::

Custom Search