Biology Reference
In-Depth Information
about chemical bonds, and one for nonbonded interactions. Nonbonded
interactions involve atoms separated by more than three chemical
bonds or atoms belonging to different molecules. These interactions are
of three types, electrostatic, short-ranged repulsion, and long-ranged
dispersion. The electrostatic interactions are usually approximated by
assuming that each atom in the system has a partial positive or nega-
tive charge. The short-ranged repulsion and long-ranged dispersion
interactions are usually approximated together with a function that
has a Lennard-Jones, or 6-12, form. In this functional form, the
r
−12
term describes the repulsion and the
r
−6
term describes the attraction.
The terms of a typical intermolecular force field and the corresponding
potential energy expressions are illustrated in figure 3.3.
In these expressions,
r
ij
represents the distance between atoms
i
and
j
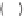
, q
ijk
represents the angle formed by atoms
i
,
j
, and
k
, f
ijkl
represents the
torsion angle formed by atoms
i
,
j
,
k
, and
l
. A force field has a large
number of parameters. The bond parameters, for example, include
the bond spring constants,
k
ij
, and equilibrium bond lengths,
r
0
. These,
in turn, depend on what are the atom types of atoms
i
and
j
, as well as
the nature of the chemical bond between them.
Force field expressions can have various amounts of complexity and
can include physical effects (not included in the expressions in figure 3.3)
Figure 3.3
Intramolecular interactions and corresponding potential energy
terms in a typical molecular mechanics force field.




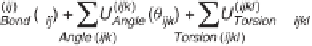












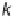








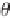

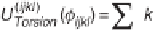




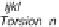


























Search WWH ::

Custom Search