Environmental Engineering Reference
In-Depth Information
(a)
(b)
(c)
Figure 7.10.
Interaction of (a) repulsive and (b) Van der Waals attractive forces to give (c) curves of net
energy of repulsion or attraction (Adapted from Mitchell, 1976).
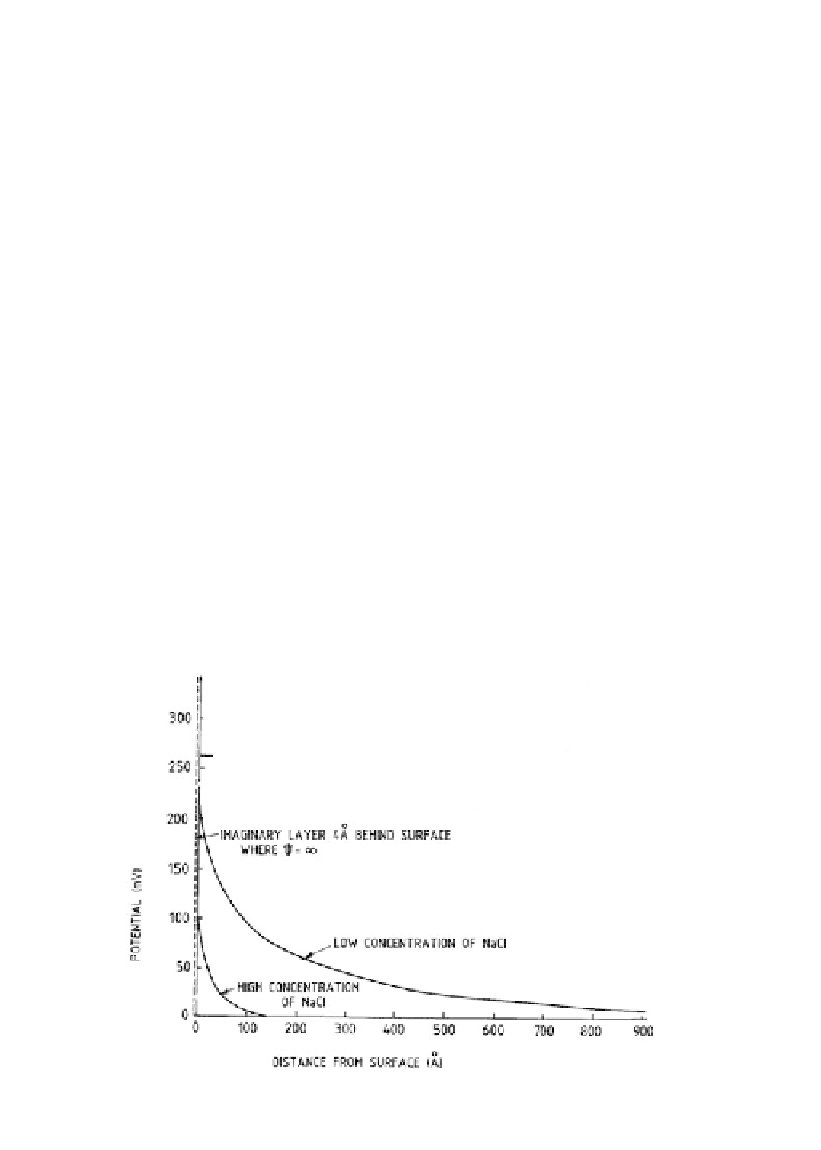
Figure 7.11.
Effect of electrolyte concentration on diffuse double layer potential for montmorillonite
(Adapted from Mitchell, 1976).
than the Van der Waal's forces the soil will disperse. In cases where the repulsive forces are
small, the Van der Waals attractive forces dominate and flocculation results.
The repulsive forces in the diffuse double layer are affected by several factors:
(a)
Electrolyte concentration
: As shown in Figure 7.11, a high concentration of dissolved salt
in the soil water leads to a smaller diffuse double layer (as the greater concentration