Environmental Engineering Reference
In-Depth Information
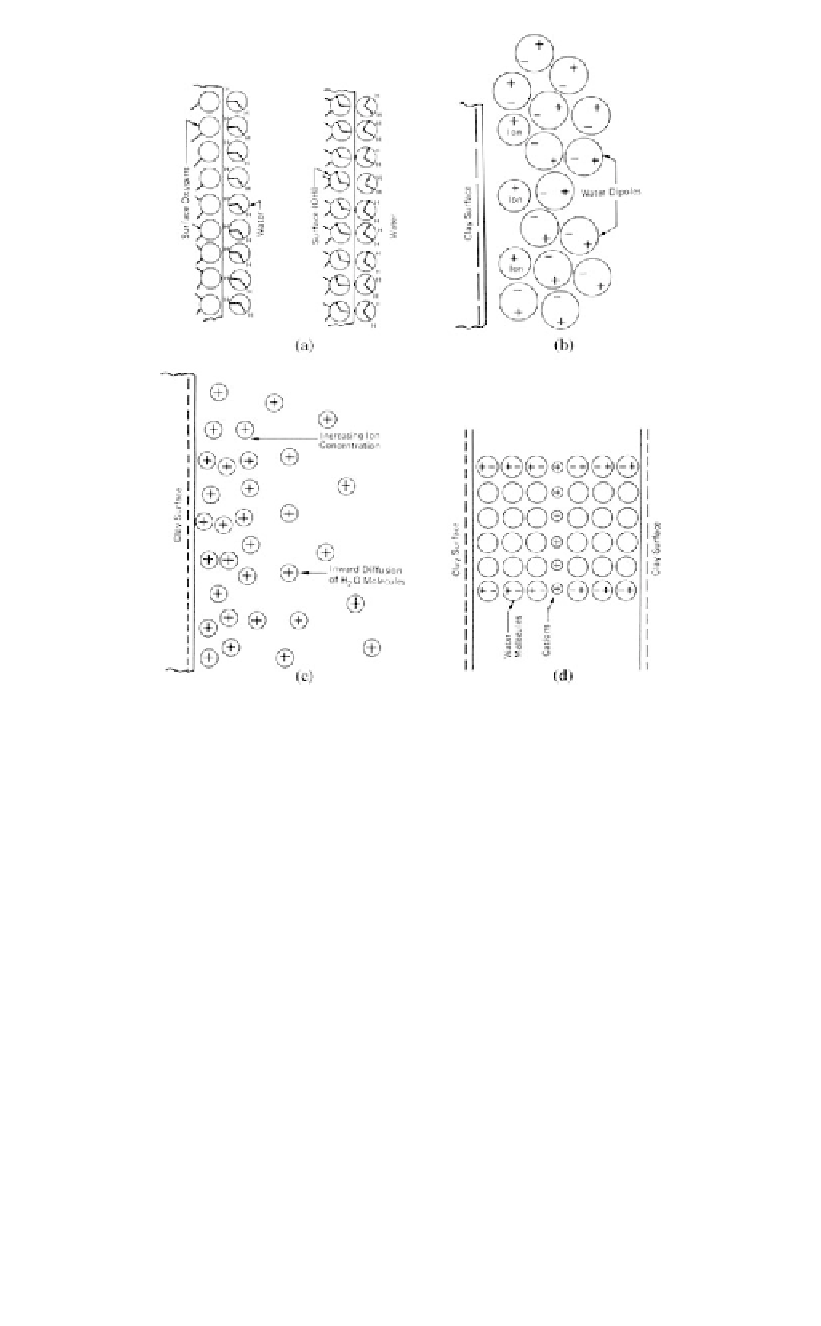
Figure 7.6.
Possible mechanisms of water adsorption by clay surfaces: (a) hydrogen bonding;
(b) ion hydration; (c) attraction by osmosis; (d) dipole attraction (Mitchell, 1976).
-
hydrogen bonding with polar water
- as shown in Figure 7.6;
-
exchangeable cation bonding
- cations, eg. Ca
, Na
act to bond between the nega-
tively charged surfaces of such clay minerals as montmorillonite. The negative charge
is brought about by substitution of cations within the sheet structure e.g. Mg
for
Al
. This is known as isomorphous substitution and is permanent. The interlayer
cations are not permanent and may be substituted by other cations;
interlayer cation bonding
- cations such as K
, Mg
, Fe
which fit in the space
between layers giving a strong bond. Examples are micas and chlorites. These cations
are not affected by the presence of water.
Figure 7.5
shows the structure of illite and
chlorite.
-
Table 7.1
lists the predominant bonding for the common clay minerals.
7.3
INTERACTION BETWEEN WATER AND CLAY MINERALS
7.3.1
Adsorbed water
There is much evidence that water is attracted to clay minerals, e.g.:
-Dry soils take up water from the atmosphere, even at low relative humidity;