Environmental Engineering Reference
In-Depth Information
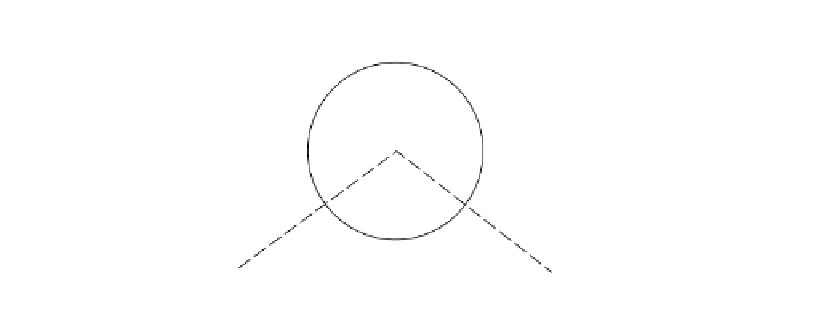
O
H
H
105º
Figure 7.4.
Schematic diagram of a water molecule showing its dipole nature (Holtz and Kovacs, 1981,
reproduced with permission of Pearson Education).
7.2.2.1
Primary bonds
These are either:
(i)
Covalent bonds
, where two atomic nuclei share one or more electrons, e.g.
H•
H
:
H, or the hydrogen molecule;
(ii)
Ionic bonds
, which result from the electrostatic attraction between positive and neg-
ative ions (ions are free atoms which have gained or lost an electron). Positive
charged ions are known as cations, negatively charged as anions;
When an ionic bond forms, the centres of negative and positive charge are sepa-
rated and form a dipole. The dipole then has a positive and negatively charged “end”,
even though it is neutral overall;
•H
Na
Cl
NaCl
(7.1)
Water is an example of a molecule which forms a dipole as shown in Figure 7.4. This
dipolar nature has an important effect on the behaviour of water in clays;
Mitchell (1976) indicates that purely ionic or covalent bonds are not common in
soils, and a combination is more likely. Silica (SiO
2
) is partly ionic, partly covalent;
(iii)
Metallic bonds
, which are of little importance in the study of soils.
7.2.2.2
Secondary bonds
(i)
Hydrogen bonds
. Attraction will occur between the oppositely charged ends of per-
manent dipoles. When hydrogen is the positive end of the dipole, the resulting bond
is known as hydrogen bonding;
(ii)
Van der Waal's bonds
. As the electrons rotate around the nucleus of an atom there
will be times when there are more electrons on one side of the atom than the other,
giving rise to a weak instantaneous dipole. This will not be permanent in one direc-
tion. The attraction of the positive and negatively charged ends of the fluctuating
dipole causes a weak bond known as Van der Waal's bond. These bonds are additive
between atoms, and although weak compared to hydrogen bonds, they decrease less
with distance from the nucleus, than the latter.
7.2.3
Bonding between layers of clay minerals
Bonding between layers of clay minerals occurs in five ways:
-
Van der Waal's forces
- these occur in most clay minerals;
-
hydrogen bonding
, e.g. between the positive end of the OH at the base of the Al octohedra
and the negative end of the O at the top of the silica tetrahedra in kaolinite (
Figure 7.3
);