Environmental Engineering Reference
In-Depth Information
following gives an outline of the subject. For more details the reader is directed to Mitchell
(1976, 1993) and Grim (1968). Holtz and Kovacs (1981) give a useful summary of the topic.
7.2
CLAY MINERALS AND THEIR STRUCTURE
7.2.1
Clay minerals
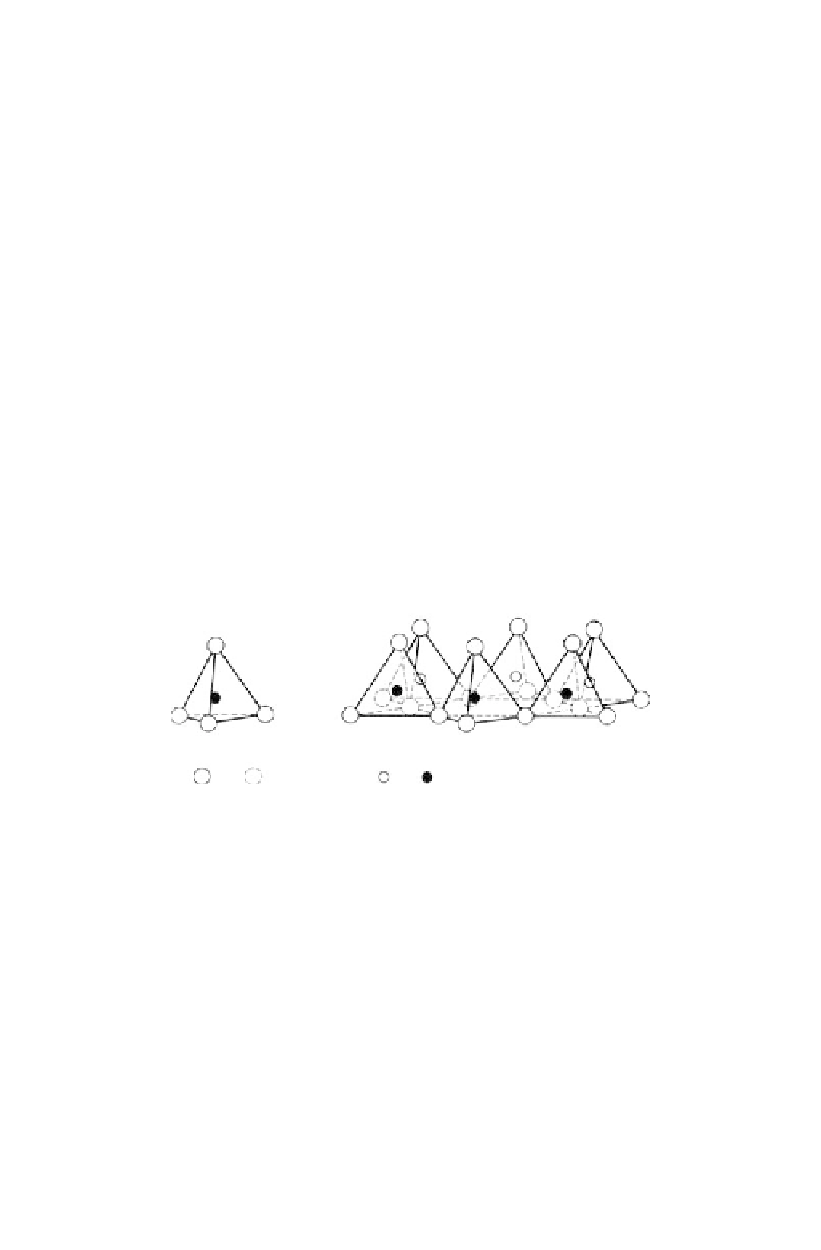
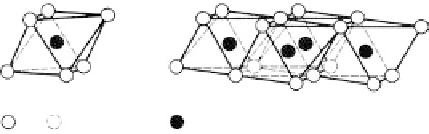
The basic “building blocks” of clay minerals are silica tetrahedra and aluminium (Al) or
magnesium (Mg) octohedra. These give sheet like structures as shown in Figure 7.2. The
alumina octohedra are known as gibbsite, the magnesium octohedra are brucite.
These in turn combine to give the clay minerals.
Figure 7.3
shows the structure of mont-
morillonite and kaolin.
Some silicate clay minerals do not have a crystalline structure, even though they are fine
grained and display claylike engineering properties. These are known as allophane and
are present in most soils. Mitchell (1976) indicates they are particularly common in some
soils formed from volcanic ash because of the abundance of “glass” particles.
Oxides also occur widely in soils and weathered rock as fine-grained particles which
exhibit claylike properties.
Examples are:
-
Gibbsite, boehmite, haematite and magnetite (oxides of Al, Fe and Si which occur as
gels,precipitates or cementing agents);
(a)
(b)
and
Oxygens
and
Silicons
Si
Si
(c)
(a) Single silica tetrahedron, (b) Isometric view of the
tetrahedral or silica sheet, (c) Schematic representation
of the silica sheet.
(a)
(b)
and
Hydroxyls or
oxygens
Aluminums, magnesiums, etc.
Al
Al
(c)
(a) Single aluminium (or magnesium) octahedron, (b) Isometric
view of the octahedral sheet, (c) Schematic representation of the
octahedral or alumina (or magnesia) sheet.
Figure 7.2.
Silica tetrahedra, and aluminium and magnesium octohedra (Holtz and Kovacs, 1981,
reproduced with permission of Pearson Education).