Environmental Engineering Reference
In-Depth Information
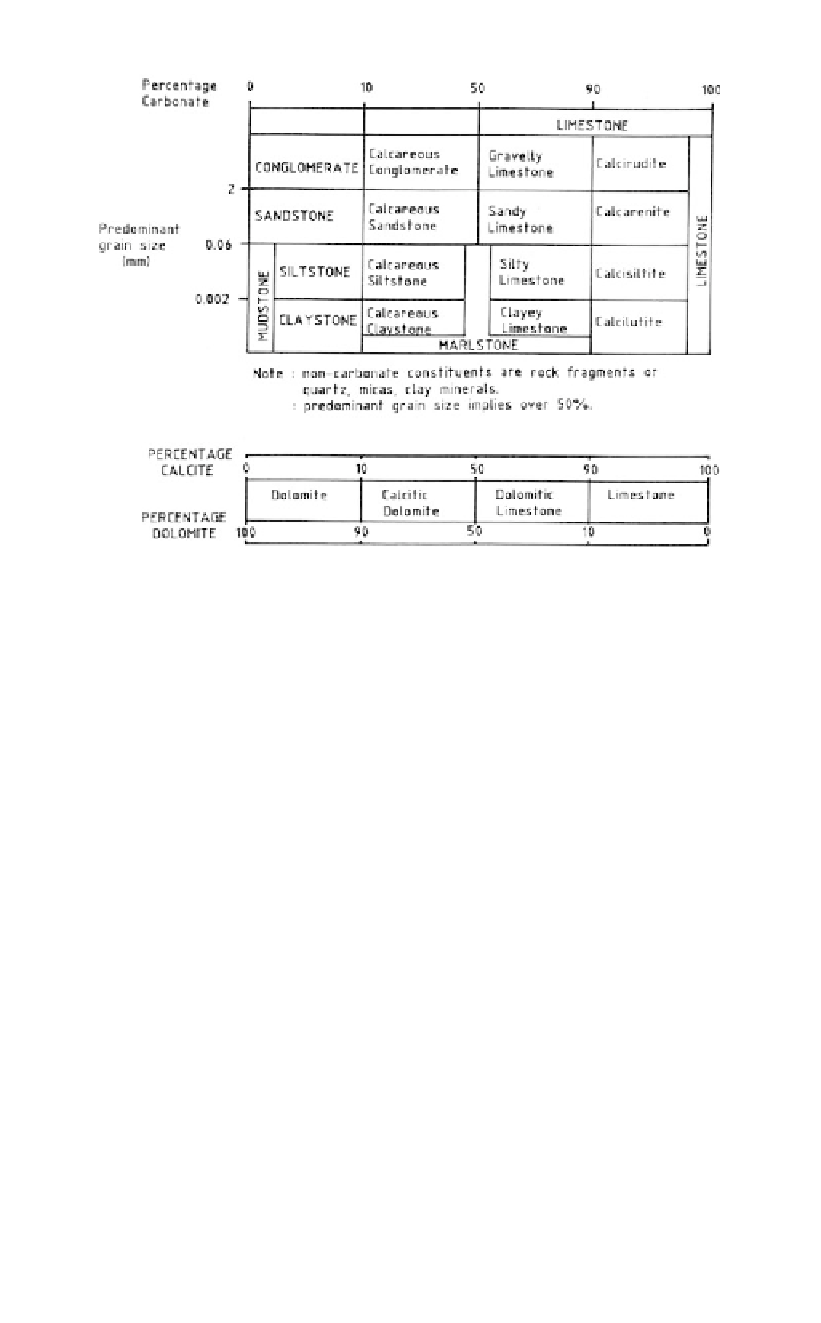
Table 3.2.
Engineering classification of sedimentary carbonate rocks (Dearman, 1981).
original pure carbonate sediments (detrital, chemical and biochemical) and their progres-
sively strengthened counterparts. This table has useful descriptive terms and its accompa-
nying discussion indicates some of the complexities involved in the strengthening of
carbonates.
Calcrete (caliche) is a
Category Y
carbonate rock of highly variable strength found in
many arid areas. The most common variety, pedogenic calcrete, occurs within a metre or
so of the ground surface (Netterberg, 1969, 1971; James and Coquette, 1984; Meyer,
1997).
3.7.1
Effects of solution
Solution is one of the processes of chemical weathering which affects all rock types to
some extent. It is more severe and causes cavities in carbonate rocks because calcite and
dolomite are relatively soluble in acidic waters e.g. carbonic (dissolved carbon dioxide),
organic (from vegetation) or sulphuric (from volcanic activity or oxidation of sulphides).
They are more soluble in saline water than in pure water and their solubility increases
with desceasing water temperatures. Also, their rates of solution are high (James and
Kirkpatrick, 1980; James, 1981).
The term karst is used to describe terrain underlain by cavernous rocks (usually car-
bonates or evaporites) and also to describe the cavernous rocks themselves. The factors
involved in the formation of karst are the same as those which contribute to the develop-
ment of weathered profiles described in Chapter 2, Section 2.6.4. Detailed discussion of
karst development, structure and hydrogeology can be found in Sweeting (1972, 1981),
Milanovic (1981), Bonacci (1987), Ford and Williams (1989), Beck (1993, 1995) and De
Bruyn and Bell (2001).
The effects of solution as seen in the most common types of carbonate rock masses are
discussed below.