Environmental Engineering Reference
In-Depth Information
9.2.6.5. Molybdate
Molybdate is extremely important in biological nitrogen fixation. Molybdate is present in
relatively low concentrations in soil, however. Acid soils with appreciable leaching may
have total molybdate levels around 0.5 mg/ kg. Basic soils having low rainfall may have
levels 10 times that amount. Soils to which sewage sludge has been added may have
significant increases in molybdate concentration. Because of its negative charge
molybdate would be expected to move readily through the environment. It reacts with
many constituents, however, and thus does not move as readily as expected [20].
9.2.6.6. Borate
Boron is present in soil and is taken up by plants as HsBO3. It can also occur as borate
and however. The boron oxyanions are generally present in very low
concentrations in soil. Boron can move by both mass flow and diffusion in the soil
solution. Because it is both an essential nutrient for and is toxic to plants it is of particular
environmental concern. There is a very narrow range over which it is sufficient but not
toxic. High levels of boron in soil can result from coal ash disposal and represent a cause
for concern when revegetation and bioremediation are undertaken [20].
9.3.
THE ORGANIC COMPONENT
Organic compounds in the environment range from the very simple to the very complex.
From methane, which is released during anaerobic decomposition of organic matter, to
humus, which is the organic matter that is produced during organic matter decomposition
and remains afterwards. Compounds of intermediate complexity are also formed during
the organic matter decomposition process. All organic functional groups can be found in
the organic components in the environment, and all of these are in addition to inorganic
elemental carbon, carbon dioxide, and carbonate.
To be an organic compound a molecule must be made up of carbon and hydrogen. It
may also contain several other different kinds of atoms, the most important of which are
oxygen and nitrogen. Other atoms, however, are common, such as sulfur, phosphorus,
and the halogens, particularly chlorine and bromine. When these other atoms are present,
their position and arrangement in the molecule are extremely important. They form the
functional groups that control a compound's interaction with the environment. Solubility
in water, movement, reactions, and toxicity are all related to a compound's functional
groups.
Halogens substituted on any organic compound have a dramatic effect on both their
physical and chemical properties. Halogens are heavy, and substituting a halogen for a
hydrogen on an organic compound increases the compound's density, sometimes making
it denser than water. Halogens also make the compounds less soluble in water. On the
other hand, many oxygen—and nitrogen-containing functional groups increase a
compound's solubility in water.
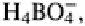
Search WWH ::

Custom Search