Environmental Engineering Reference
In-Depth Information
both solid inorganic surfaces and organic components, and thus are also removed from
solution by these mechanisms. For these reasons phosphate does not typically move in
soil unless unusual conditions, such as extremely high levels of phosphate or very sandy
soils, occur [18].
High levels of plant-available phosphate in soil and water can occur naturally. In
Florida, where phosphate is mined and soils are sandy, soil phosphate can be so high that
it readily leaches into water. In any area in which phosphate is mined the Earth's surface
may have high concentrations of phosphate. Both of these cases are easily explained.
Such situations are unusual, however, and any time high levels of phosphate are found
the source of phosphate needs to be determined.
9.2.6.4. Oxides of Nitrogen
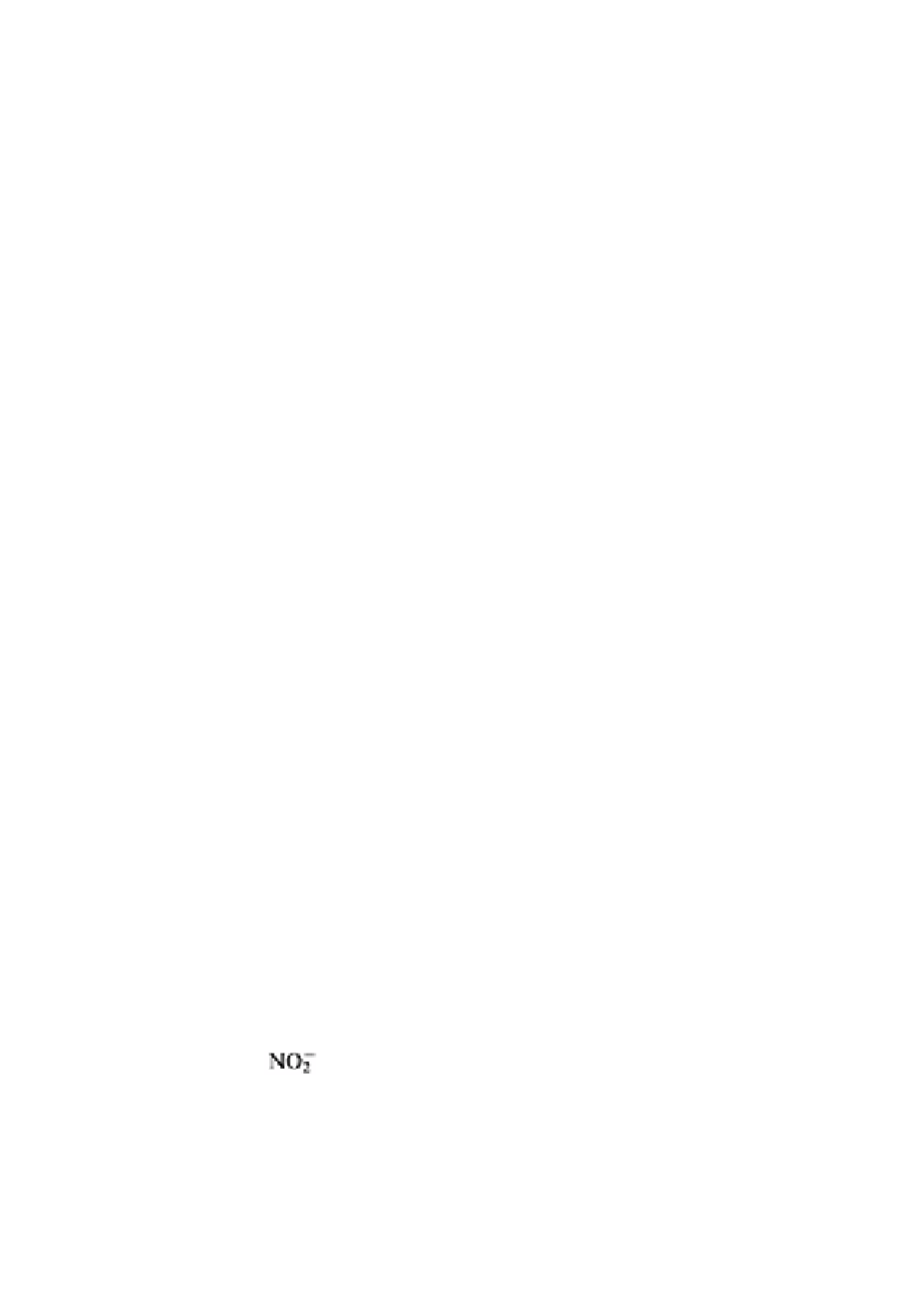
The two most common oxides of nitrogen are the oxyanions nitrite and nitrate. Table 9.3
provides a list of all the common environmental oxides of nitrogen. Nitrogen is added in
large amounts to agricultural land as nitrogen fertilizer, anhydrous ammonia, aqueous
ammonia solutions, and various nitrates, including ammonium nitrate. Ammonium added
to the soil when temperatures are above 5°C and in the presence of moist but not
saturated water conditions is quickly oxidized to nitrite and then nitrate. Nitrite is usually
present in small amounts because oxidation of nitrite to nitrate is faster than the oxidation
of ammonia to nitrite. During the oxidation of ammonia to nitrate protons are released
into the surroundings. Thus both soil and water may become acidic during this
transformation.
Both nitrite and nitrate carry a negative charge. They are mobile in soil, and their
compounds are very soluble. When either nitrite or nitrate are present in soil they can
leach into the ground water. However this only occurs as long as they are under oxidizing
conditions. Under reducing conditions nitrate is reduced to N
2
, NO, and N
2
O gases. The
occurrence of nitrate along with the various oxides of nitrogen in environmental samples
should be expected. When they are found, increased acidity may also be found. The
occurrence of large amounts of nitrite in environmental samples indicates an unusual
situation that needs further investigation [19].
TABLE 9.3
Common Environmental Oxides of Nitrogen
Name
Chemical formula
Common occurrence
Nitric oxide
NO
During denitrification
N
2
O
Nitrous oxide
During denitrification
NO
2
Nitrogen dioxide
During denitrification
Nitrite
First oxidation product of ammonia
Nitrate
Final product in oxidation of nitrite



Search WWH ::

Custom Search