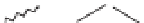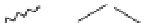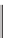Agriculture Reference
In-Depth Information
CH
2
OH
CH
2
OH
O
H
O
H
H
OH
H
O-
-OH
OMe
OMe
O
O
CH
2
OH
CH
2
OH
H
OH
H
O
H
H
OH
H
2
O
O-
+
OMe
OMe
O
O
Fig. 13.7.
Hydrolysis of the lignin structure by sodium hydroxide.
-SH
+ MeSH
O
O-
O
3
OH
OH
O
ortho-quinone
Fig. 13.8.
Generation of chromophoric compounds responsible for pulp colour.
13.4.4
Acidic sulfite process
(Na
2
SO
3
) as a reagent. The chemical reactions
are intended to achieve limited delignification
through the combined effects of sulfonation
and hydrolysis (sulfitolysis). High reaction tem-
peratures (160-190°C) are used to accelerate
sulfonation.
A near neutral pH is used in cooking to
minimize carbohydrate loss, and the cook-
ing liquor has a high buffering capacity
(bicarbonate-carbonate) to compensate for
pH drops caused by the formation of free
acids through the decomposition of
hemicelluloses.
Sulfite pulping derives its name from the use of
a bisulfite solution as the delignifying medium.
The cations used are generally calcium,
magnesium, sodium or ammonium. The bisulfite
process operates at pH 2-3. The bisulfite
solution is strengthened by the addition of
SO
2
gas.
The cooking liquor is characterized as follows:
•
Cooking liquor components (total SO
2
): M
2
SO
3
+ H
2
SO
3
+ SO
2
, where M is the cation