Biology Reference
In-Depth Information
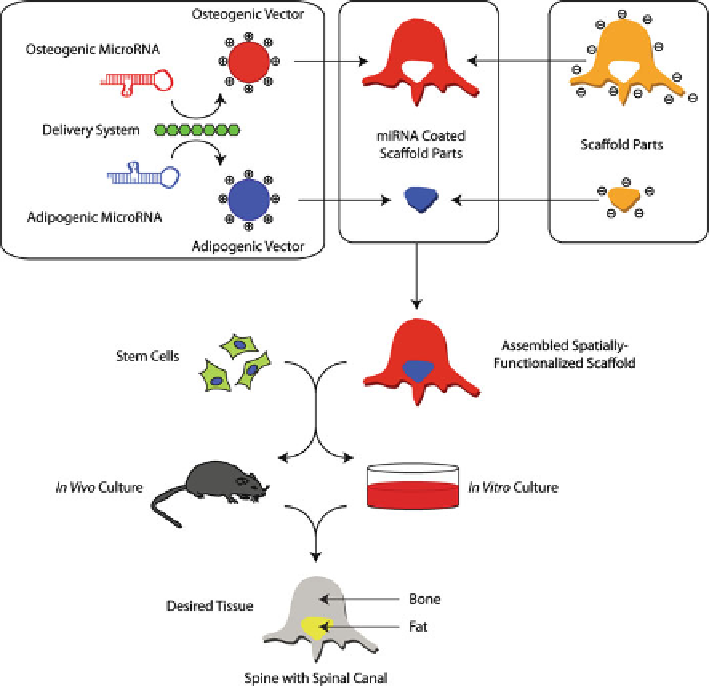
Fig. 3.3
Shows a modification of the strategy previously used to create a spatially differentiated
tissue [
25
]. First, osteogenic and adipogenic miRNA modulators are incorporated into delivery
vectors; these are then coated onto scaffold parts with the shape of the tissue section they will cre-
ate. The miRNA vectors interact with the surface through van der Waals and ionic bonding, bind-
ing them to that specific scaffold part. The scaffold components are then assembled in the correct
form, and stem cells added. During further in vitro or in vivo culture, the cells take up the miRNA
from their local environment, and the miRNA induces them to locally create the correct tissue.
Figure modi fi ed from [
6
]
The main advantage of implant-mediated miRNA delivery is the ability to control
spatial-temporal miRNA transfection. Our lab has previously devised a method for
delivering two different siRNAs from the surface of different parts of an implant [
25
] .
Using TransIT-TKO, we produced nanoparticles containing siRNAs against BCL2L2
and TRIB2, proteins that inhibit osteogenesis and adipogenesis, respectively. These
cationic nanoparticles could be adsorbed onto anionic nanoporous polycaprolactone
scaffolds prior to seeding with MSCs. After implantation, the siRNAs guided the
development of two distinct tissues at discrete locations. The same system could in
principle be used for delivering miRNA or anti-miRNA in specific regions of an
implant (Fig.
3.3
). Instead of using siRNAs against BCL2L2 and TRIB2 to promote
Search WWH ::

Custom Search