Biology Reference
In-Depth Information
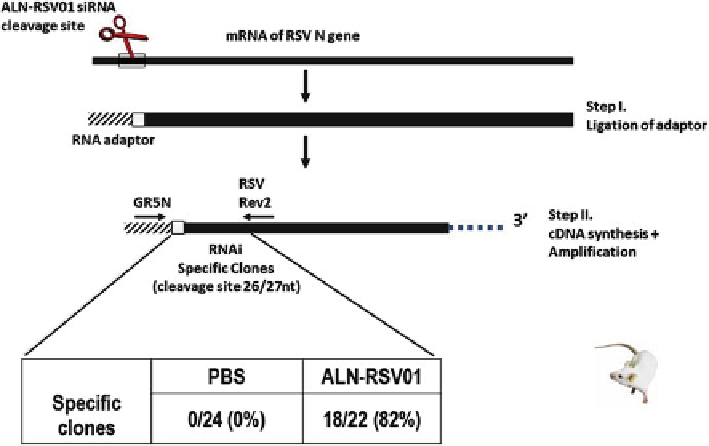
Fig. 15.2
ALN-RSV01 antiviral activity is mediated by RNAi in vivo. Shown is a schematic
representation of the 5¢ RACE assay used to demonstrate site-specific cleavage product. The
results from the sequence analysis of individual clones from PCR amplification of cDNA gener-
ated from linker-adapted RSV N-gene mRNA isolated from an in vivo experiment are shown in
box
. In this experiment, mice were treated with ALN-RSV01 or PBS (negative control) 3 days
after infection. Lungs were harvested 5 days after RSV01 treatment for RACE analysis
the clones isolated from lung tissue of ALN-RSV01 treated, RSV-infected mice
demonstrated site-specific cleavage of the specific N-gene transcript, while animals
treated with PBS did not (Fig.
15.2
) [
22
] .
15.2.5
Preclinical Toxicology
A comprehensive set of preclinical repeat-dose toxicology studies via intranasal
(i.n.), inhalation, and intravenous (i.v.) routes was performed with ALN-RSV01 in
rat and cynomolgus monkey (unpublished data). No significant drug-related toxici-
ties were seen in either species across a range of ALN-RSV01 doses. PK data from
the inhalation studies in monkeys showed short-lived, low-level plasma concentra-
tions of ALN-RSV01 postdose, consistent with very low systemic exposure follow-
ing inhalation due to rapid degradation and clearance of siRNA entering the
circulation. Similarly, i.v. injection in monkeys also demonstrated no significant
toxicities and a short plasma half-life, which is consistent with the short in vitro
half-life in human serum (~13 min) (unpublished data).
Search WWH ::

Custom Search