Agriculture Reference
In-Depth Information
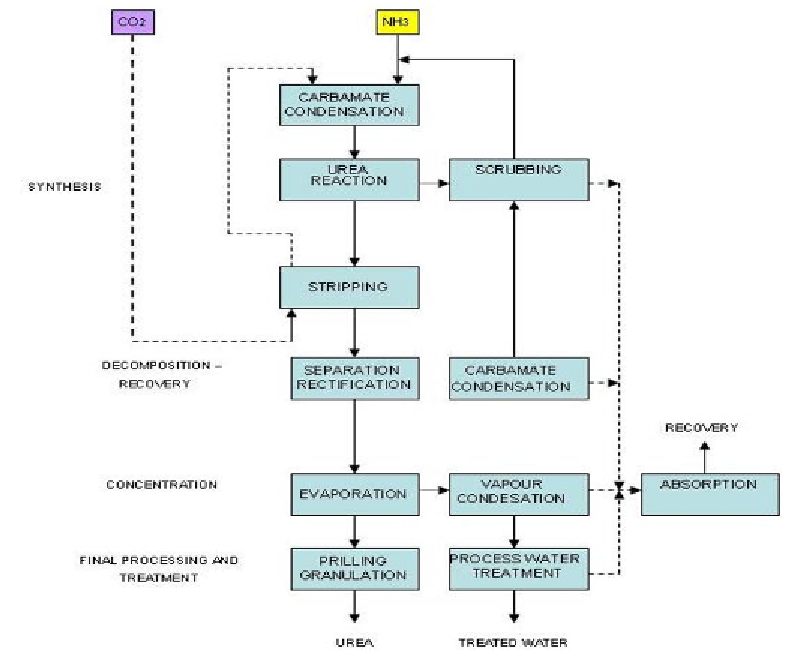
Figure 4. Block Diagram of a Total Recycle NH
3
Stripping Urea Process (EFMA, 2000a).
UAN is produced from urea and ammonium nitrate trough continuous or batch type
processes in which concentrated urea and ammonium nitrate solutions are measured, mixed
and then cooled. A partial recycling CO
2
stripping urea process is also suitable for UAN
solution production. The GHGs emission from UAN production is dominated by N
2
O
emission because nitric acid is an intermediate product in ammonium nitrate synthesis.
Ammonium Nitrate (AN) and Calcium Ammonium Nitrate (CAN)
Ammonium nitrate and calcium ammonium nitrate are the most used N-fertilizers in
Europe. Ammonium nitrate is made by neutralizing nitric acid with anhydrous ammonia
(European Commission, 2006; EFMA, 2000a):
NH
3
+ HNO
3
NH
4
NO
3
.
The reaction is highly exothermic and proceeds rapidly. The heat produced is often used
to generate steam. The steam in turn may be used to evaporate the ammonia. The resulting
80-90% solution of ammonium nitrate can be sold as it is or it may be further concentrated to
a 95-99.5% solution (melt) and converted into prills or granules. The manufacturing steps
Search WWH ::

Custom Search