Biology Reference
In-Depth Information
Physical medium
(air, waters, soils or sediment)
Organism
Potential risk
Radionuclides
Toxic effect
Bioaccumulation
Exposure
Excretion
Stockage
BARRIERS
Increased toxicity
(reactive metabolites)
Physical
dilution
Detoxification
- Biomineralization
- Metallothioneins
Biotransformation
- Phase I oxidation
- Phase II conjugation
Chemical
transformation
Biological
membranes
Tolerance patterns
- Physiological acclimation
- Genetic adaptation
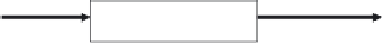
FIGURE 1.1
The ecotoxicology triad.
their metal binding characteristics and consequently the biological uptake of the metal.
Another mechanism of limiting contaminant uptake is the existence of impervious extra-
cellular barriers such as cuticles, integuments, tests, shells, and scales that contribute to
reduce the cell epithelial surface available to contribute to transepithelial transport (for
details, see Mason and Jenkins 1995).
Once incorporated into an organism (Figure 1.1), contaminants can be either stored in
tissues or excreted. Storage in intra- or extracellular compartments does not necessar-
ily result in a toxic effect in organisms. For instance, metal detoxification is efficient in
numerous organisms. It may be based on the synthesis of metallothioneins (MTs), a fam-
ily of metalloproteins able to sequester metals via metal binding to their constituent thiol
groups, thus blocking any interference between the metals and enzymes that would oth-
erwise result in subsequent enzymatic activity impairments. MT induction is the most
common toxic metal defense mechanism in vertebrates. It is also present in most biological
taxa (Amiard et al. 2006), but among invertebrates, the major mode of metal detoxification
is metal biomineralization in various types of cellular inclusions (Marigomez et al. 2002).
It is only when the metal-binding capacity of these ligands is overwhelmed that metal
toxicity can occur.
On the contrary, processes responsible for excretion are not systematically free of noxious
effects on organisms. Biotransformation of certain organic pollutants [polycyclic aromatic
hydrocarbons (PAHs), PCBs] is organized into two phases (Figure 1.1). Phase I reactions
consist of oxidation, reduction, and hydrolysis processes. Phase II enzymes serve to link
metabolites from phase I with endogenous substrates, increasing their water solubility
and thereby facilitating their excretion. However, phase II biotransformation sometimes
leads to reactive metabolites, the interactions of which with cellular macromolecules can
engender toxicity (Roméo and Wirgin in Amiard-Triquet et al. 2011). Biotransformation
is followed by phase III leading to the elimination of metabolites by the multixenobiotic
transport system (Damiens and Minier in Amiard-Triquet et al. 2011).
The activity of biotransformation enzymes (such as cytochrome P450 enzymes, including
ethoxyresorufin
O
-deethylase involved in phase I; glutathione
S
-transferase involved in
phase II) or MT concentrations are some examples of biomarkers that have been proposed












Search WWH ::

Custom Search