Environmental Engineering Reference
In-Depth Information
Q
Out
Condenser
Generator
Q
In
W
In
Expansion
valve
Throttling
valve
Q
Out
Evaporator
Absorber
Q
In
Thermal
compression
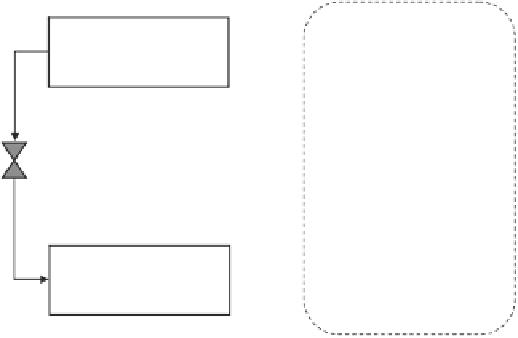
Figure 11.11
Basic absorption refrigeration cycle.
Adapted from Dinçer, 2003.
refrigerant and water is the absorbent. Water-lithium bromide can be used only for temperatures
above 0°C, otherwise water freezes; and ammonia-water can be used for subzero applications.
When the refrigerant gets absorbed into the absorbent there is a release of heat that needs
removal. In single-effect absorption systems heat is rejected to the environment. Instead, this
heat can be used to partially heat the generator of a second effect thus increasing the efficiency
of whole systems.
The coefficient of performance (defined as the energy consumed to remove a certain
amount of heat from a low-temperature source) is around 0.7 for a single-effect system and
varies from 1.0 to 1.2 for a double-stage system (Dinçer, 2003). In contrast, coefficients of
performance for single-stage vapor compression cycles range between 0.8 and 2.5 and from
1.2 to 2.7 for two-stage compressors (Valentas et al., 1997).
Even when absorption systems have lower coefficients of performance than vapor com-
pression systems, absorption systems have the advantage of being able to use low-temperature
waste process heat or heat from CHP systems, which reduces the total energy requirements.
When in addition to power and heat, cooling produced in CHP systems is generally referred
as
trigeneration
.
The description presented in this section is a simplified version that shows the principles of
absorption cycles. Commercial systems are much more complex, and readers with interest in
those systems can consult specialized literature.
SUMMARY
Energy is absolutely essential for human activities. The development of society to levels never
seen before is in great part due to the availability of inexpensive fossil fuels. So for long-term
sustainability it is crucial to find ways to use energy more efficiently and to develop alternative
energies to substitute current fossil fuels.