Biology Reference
In-Depth Information
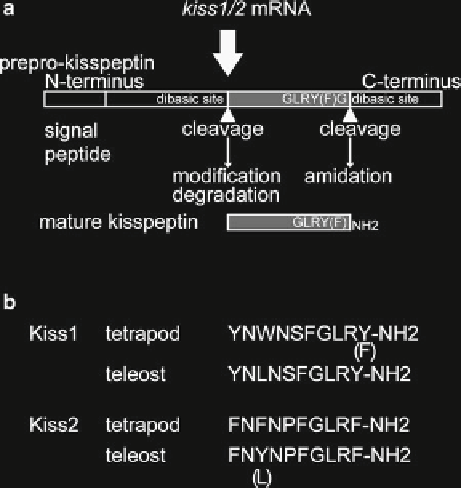
Fig. 2.1
Schematic illustration of Kiss1/Kiss2 peptide maturation process and conserved peptide
core sequence in vertebrates. (
a
) Prepro-kisspeptin molecules are cleaved into shorter kisspeptin
by the dibasic cleavage sites. The C terminus of kisspeptin is amidated to the characteristic RF or
RY motif. After cleavage, amidation occurs at C terminus, and degradation and/or modifi cation
such as pyroglutamate formation may occur at N terminus. (
b
) A summary of core sequence of
Kiss1 and Kiss2 in vertebrates. Note that tyrosine and tryptophan possess similar side-chain
brains [
3
]. From the prediction of a cleavage site and subsequent binding assay
studies, it has been shown that the 10 amino acid “core sequence” is essential and
suffi cient for the full activation of Gpr54 by Kiss1 and Kiss2 throughout vertebrates
in general [
3
-
5
] (Fig.
2.2
). Consequently, many researchers refer to the peptides that
possess the highly conserved 10 amino acid core sequence as “kisspeptins” and
have used kisspeptin-10 as kisspeptin in many studies. However, not many studies
have purifi ed “native” forms of kisspeptins in various vertebrate species, and we
should therefore be careful about the interpretation of physiological experiments
using only the kp-10 as kisspeptin ligands, since there may be some other physio-
logical functions that are slightly different when conveyed by the natural peptides.
Evolution of Kisspeptins and Their Receptors
Phylogeny of Kiss1 and Kiss2 Genes
For proteins that possess longer amino acid residues, sequence similarity of proteins
can be used for the construction of phylogenetic trees rather easily. However, shorter
Search WWH ::

Custom Search