Biology Reference
In-Depth Information
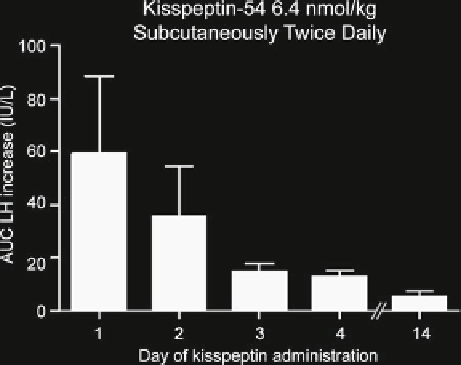
Fig. 5.4
Desensitization
induced by chronic
administration of kisspeptin
to women with hypothalamic
amenorrhea. Women with
hypothalamic amenorrhea
(
n
= 10) exhibited
desensitization to kisspeptin
when given kisspeptin-54
6.4 nmol/kg subcutaneously
twice daily. Error bars
indicate SEM. Adapted from
Jayasena et al. [
63
], with
permission from Nature
Publishing Group
no longer elicited a detectable LH response. The response to exogenously adminis-
tered GnRH was intact, suggesting that continuous exposure to kisspeptin caused
desensitization at the level of the kisspeptin receptor [
43
]. A subsequent study
detailed the time-course of this phenomenon and demonstrated that responses to
kisspeptin decreased rapidly during the fi rst few days of treatment (Fig.
5.4
) [
63
].
Because subcutaneous administration of kisspeptin-54 6.4 nmol/kg results in
sustained elevation of kisspeptin immunoreactivity lasting at least 4 h [
15
], it is
likely that twice-daily dosing resulted in continuous exposure to kisspeptin. As
noted previously, continuous exposure to kisspeptin results in desensitization of
the kisspeptin receptor in a variety of experimental models [
25
-
29
], and a similar
phenomenon appears to have occurred in this study. However, the time course of
desensitization in humans was distinct, occurring over days instead of hours [
63
].
To avoid inducing desensitization, the same group employed twice weekly (instead
of twice daily) subcutaneous administration of kisspeptin-54 6.4 nmol/kg [
63
]. After 8
weeks on this regimen, responses to kisspeptin were still seen, though they were slightly
dampened compared to the responses on Day 1. Despite the fact that the gonadotropin
responses to kisspeptin remained intact during the 8-week study, no folliculogenesis
was observed on ultrasound, and ovulation was not achieved [
63
]. Because kisspeptin
secretion is itself pulsatile [
64
,
65
], rescue of reproductive endocrine activity in women
with HA may require pulsatile delivery of kisspeptin, much as exogenous GnRH must
be delivered in a pulsatile fashion to stimulate the reproductive endocrine axis [
66
].
Safety of Kisspeptin in Humans
Collectively, published reports of kisspeptin administration to men and women have
reported no adverse events, no subjective complaints, no changes in blood pressure
or other vital signs, and no changes in blood cell counts or tests of kidney or liver
function [
11
,
13
-
15
,
24
,
31
,
43
,
63
,
67
].
Search WWH ::

Custom Search