Environmental Engineering Reference
In-Depth Information
the surface by reducing the internal resistance; and (d) reducing charge carriers travel
time by decreasing the particle size or thin film thickness. In this section, we will
discuss two of the possible ways to modify the photoactivity, namely, particle size
control and the impurity doping.
Other criteria to be considered when choosing an ideal water purification
photocatalyst are: (a) thermodynamically stable in the presence of water; (b) the energy
level of the valance band edge being well below the chemical potential of O
2
/H
2
O [i.e.,
1.3 v normal hydrogen electrode (NHE)] to prevent the corrosion of photocatalysts and
maintain a high oxidation potential in the reaction; (c) low cost for synthesis; and (d)
the bandgap of the photocatalyst being as low as possible in addition to conditions
(a)(3) above being satisfied.
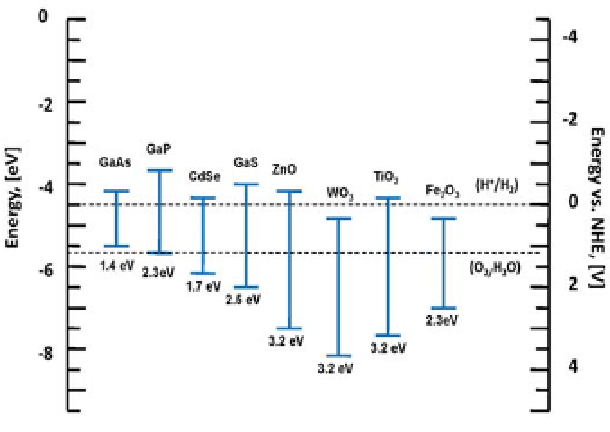
Many semiconductors have been studied intensively for the environmental
cleaning and photovoltaic applications for decades. Figure 3.5 is the diagram showing
bandgap energies and band edges of different semiconductors and relative energies with
respect to the absolute vacuum energy scale (AVS) and NHE in electrolyte (at pH = 1)
(Linsebigler et al., 1995).
Figure 3.5
Diagram showing bandgap energies and band edges of different
semiconductor photocatalysts and relative energies with respect to the AVS and NHE in
electrolyte at pH = 1 (Replotted from Linsebigler et al., 1995).
Figure 3.5 shows that low bandgap semiconductors such as GaAs, GaP, and
GdSe, GaS, and Fe
2
O
3
have the valance band edges above or close to the O
2
/H
2
O redox
Search WWH ::

Custom Search