Environmental Engineering Reference
In-Depth Information
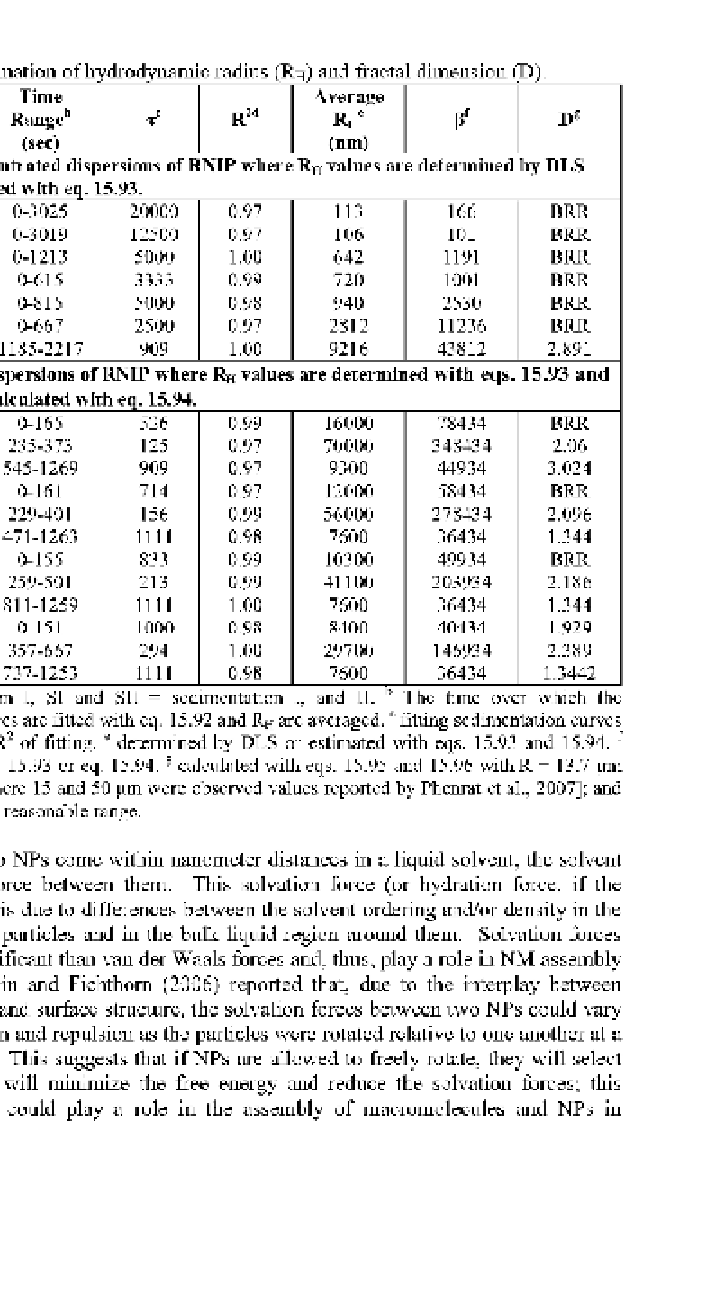
Table 15.12
Estimation of hydrodynamic radius (RH) and fractal dimension (D).
Time
Range
1
"
(sec)
Average
R
H
e
(nm)
D
g
R
2d
P'
T°
Diluted or concentrated dispersions of RNIP where R
H
values are determined by DLS
and p is calculated with eq. 15.93.
0-3025
0-3019
0-1213
0-615
0-815
0-667
1185-2217
BRR
BRR
BRR
BRR
BRR
BRR
2.891
Concentrated dispersions of RNIP where R
H
values are determined with eqs. 15.93 and
15.94 and p is calculated with eq. 15.94.
20000
12500
5000
3333
5000
2500
909
0.97
0.97
1.00
0.99
0.98
0.97
1.00
113
106
642
720
940
2812
9216
166
101
1191
1001
2530
11236
43812
BRR
2.06
3.024
BRR
2.096
1.344
BRR
2.186
1.344
1.929
2.389
1.3442
a
AI = aggregation I, SI and SII = sedimentation I, and II. The time over which the
sedimentation curves are fitted with eq. 15.92 and R
H
are averaged. ° fitting sedimentation curves
0-165
235-373
545-1269
0-161
229-401
471-1263
0-155
259-501
811-1259
0-151
357-667
737-1253
526
125
909
714
156
1111
833
213
1111
1000
294
1111
0.99
0.97
0.97
0.97
0.99
0.98
0.99
0.99
1.00
0.98
1.00
0.98
16000
70000
9300
12000
56000
7600
10300
41100
7600
8400
29700
7600
78434
348434
44934
58434
278434
36434
49934
203934
36434
40434
146934
36434
with eq. 15.92.
d
R
2
of fitting.
e
determined by DLS or estimated with eqs. 15.93 and 15.94.
f
calculated with eq. 15.93 or eq. 15.94.
6
calculated with eqs. 15.95 and 15.96 with R = 13.7 |im
[= (15*50)
1/2
/2, where 15 and 50 jim were observed values reported by Phenrat et al., 2007]; and
BRR
—
beyond the reasonable range.
When two NPs come within nanometer distances in a liquid solvent, the solvent
can mediate a force between them. This solvation force (or hydration force, if the
solvent is water) is due to differences between the solvent ordering and/or density in the
gap between the particles and in the bulk liquid region around them. Solvation forces
can be more significant than van der Waals forces and, thus, play a role in NM assembly
and stability. Qin and Fichthorn (2006) reported that, due to the interplay between
solvent ordering and surface structure, the solvation forces between two NPs could vary
between attraction and repulsion as the particles were rotated relative to one another at a
fixed separation. This suggests that if NPs are allowed to freely rotate, they will select
orientations that will minimize the free energy and reduce the solvation forces; this
direct alignment could play a role in the assembly of macromolecules and NPs in
Search WWH ::

Custom Search