Environmental Engineering Reference
In-Depth Information
trichloroethylene (TCE), tetrachloroethene (PCE) or carbon tetrachloride ( Zhang, 2003;
Nurmi et al., 2005). In addition, INPs can also remove or transform heavy metals such
as Cr(VI) (Ponder et al., 2000; Manning et al., 2006), lead, nickel or mercury (Li et al.,
2006), radionuclide such as U(VI) (Gu et al., 1998), metalloids (Kanel et al., 2005,
2006), perchlorate (Cao et al., 2005a), humic acid and nitrate (Giasuddin et al., 2007b;
Liou et al., 2006; Shin and Cha, 2008; Sohn et al., 2006) . INPs can also produce
hydroxide radicals in the presence of oxygen to oxidize a variety of organic
contaminants such as carbothioate herbicide/molinate and benzoic acid (Joo et al.,
2005). For example, the reactivity of these INPs was found to be 3 orders of magnitude
greater than micron size iron for As(III). This is mainly due to versatile reactivity of
INPs induced by continuous generation of corrosion surface by zero valent iron from the
core. Due to its typical characteristics, interaction of INP with a model contaminant can
occur by multiple mechanisms (e.g., adsorption, oxidation, reduction). Typically, TCE,
PCE can be reduced by INP whereas Cr(VI) can be reduced as well as adsorbed by INP.
However, in the case of As(III), oxidation and adsorption mechanism will be dominant
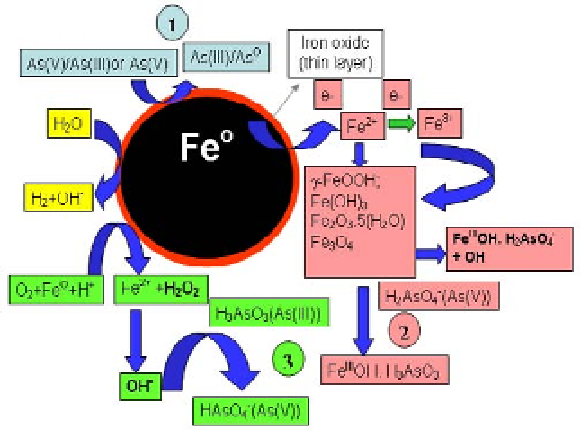
(Figure 11.8).
Figure 11.8
Mechanisms for INPs reacting with arsenic.
Kanel et al. (2005) investigated the long term interaction between INPs and
As(III) in a batch study using solid samples collected from pristine INPs and INPs
treated by 100 mg/L As(III) for 7, 30 and 60 days. Figure 11.9 shows different surface
textures and different pore sizes with respect to the adsorption time and precipitation of
As onto INPs. Aggregation of particles increases with reaction time due to Fe(III)
Search WWH ::

Custom Search