Environmental Engineering Reference
In-Depth Information
no irreversible sites on the surface of the adsorbent and suggesting a high recovery
capacity of the adsorbent. In addition to chromium, iron and manganese were measured
after each adsorption/desorption process. It was found that the concentrations of these
two metals were nearly zero, hinting that the dissolution of the nanoparticles under
stated experimental conditions was not a concern. Therefore, the stability and durability
of the adsorbent during adsorption and desorption processes were verified.
120
100
80
Adsorption capacity (mg/g)
Desorption efficiency (%)
60
40
20
0
1
2
3
4
5
6
Cycle number
Figure 9.32
Performance of modified MnFe
2
O
4
in a six-cycle regeneration study.
9.5.4 Surface-Coated -Fe
2
O
3
for Cr(VI) Removal
5 g/L of -FeOOH-coated -Fe
2
O
3
nanoparticles were shaken with 20 mL of 100
mg/L Cr(VI) for 30 minutes to reach the equilibrium. The removal efficiencies are
shown in Figure 9.33. It was observed that the removal efficiency of Cr(VI) by -
FeOOH-coated -Fe
2
O
3
nanoparticles increased with an increase in the ratio of -
FeOOH to -Fe
2
O
3
until 1.0 but leveled off with an further increase in the ratio. As a
consequence, the -FeOOH-coated -Fe
2
O
3
nanoparticles with a mass ratio of 1.0 can be
considered as the optimal adsorbent for Cr(VI) removal. To find a possible dominant
parameter determining the Cr(VI) removal efficiency, the surface area of the various -
FeOOH-coated -Fe
2
O
3
nanoparticles were measured and the data are also shown in
Figure 9.33. It was found that the surface area decreased continually with an increase in
the mass of coated -FeOOH; while the removal capacity increased despite the
decreased surface area. Thus, it can be concluded that the property of the surface coating
(-FeOOH) dominates the adsorption capacity rather than the surface area of the coated















































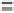









Search WWH ::

Custom Search