Environmental Engineering Reference
In-Depth Information
equilibrium time. It should be noted that since the quantity of the -Fe
2
O
3
nanoparticles
using
high-temperature decomposition of organic precursors is very small (1 g/batch)
and the producing cost is much higher compared to those synthesized using the sol-gel
method, the -Fe
2
O
3
nanoparticles produced by the sol-gel method was further used for
the following studies.
1.4
1.2
3 nm
7 nm
11 nm
15 nm
1.0
0.8
0.6
0.4
0.2
0.0
0
2
4
6
8
10
12
14
16
t (min)
Figure 9.21
Effect of contact time on Cr(VI) removal by -Fe
2
O
3
.
9.5.2.5 Adsorption Isotherms
The adsorption data of Cr(VI), Cu(II) and Ni(II) onto -Fe
2
O
3
nanoparticles were
analyzed and fitted by the Langmuir isotherm (Ce/qe vs. Ce) as shown in Figure 9.22.
The Langmuir parameters and the correlation coefficients of the adsorption data to this
equation are given in Table 9.6. The Langmuir model effectively described the
adsorption data with all R
2
> 0.98. Thus, the applicability of monolayer coverage of
heavy metals on the surface of -Fe
2
O
3
was verified. By comparison of q
m
, the
adsorption capacity of the -Fe
2
O
3
nanoparticles for heavy metals followed the
decreasing order: Cu(II)> Ni(II)> Cr(VI). The fundamental characteristics of Langmuir
equation can be interpreted in terms of a dimensionless constant separation factor (R
L
),
which is defined by:
1
bC
R
L
=
(Eq. 9.10)
1
+
0





























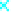





























Search WWH ::

Custom Search