Environmental Engineering Reference
In-Depth Information
9.4.4 Characterization of Metal-Doped -Fe
2
O
3
The properties of Fe oxides could be changed with increasing metal-doping. For
example, the reddish brown color of pure maghemite turned reddish yellow upon
incorporation of Al. Among these properties are the positions of the visible and infrared
absorption bands, magnetic properties, reductive dissolution, crystal size and aspect ratio.
It is, however, often difficult to assign changes in a single property unequivocally to
substitution because several properties may be modified simultaneously. The TEM
images of synthesized materials revealed that the multidispersed metal-doped -Fe
2
O
3
particles have an average diameter of around 15 nm. XRD patterns indicated that all of
them are highly crystalline. XRD results of metal-doped -Fe
2
O
3
validated that the molar
ratio of divalent metal to iron is almost equal to 1:10, which indicates that all doping
metals have been incorporated into the -Fe
2
O
3
structure. XRD results for various Al-
doped -Fe
2
O
3
confirmed the respective molar Al-Fe ratios to be 7.5%, 9.3%, 11.0% and
13.1%. By comparison, Sidhu et al. (1978) synthesized substituted Fe
3
O
4
containing up
to 0.01 transition metals at 90
o
C. Substituted Fe
3
O
4
prepared at pH 12 from ferrihydrite
contained more than 0.1 mol/mol of Mn, Cu, Co or Ni (Cornell et al., 1992; Regazzoni
and Matijevic, 1983). Up to 17 molar percentage of Al-doped -Fe
2
O
3
was found in
natural or laboratory-produced goethites (Cornell and Schwertmann, 2003).
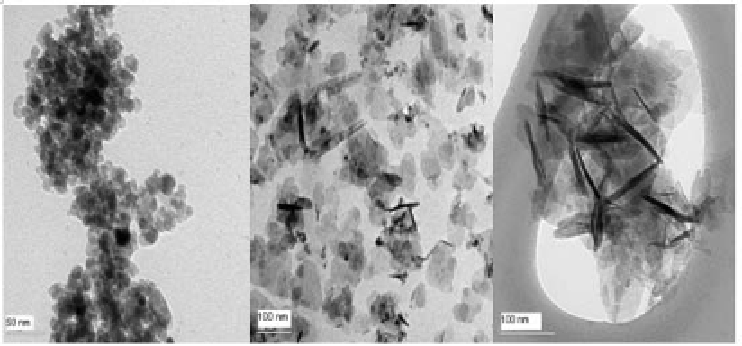
(a) (b) (c)
Figure 9.11
TEM images of (a) undoped -Fe
2
O
3
, (b) Al-doped -Fe
2
O
3
with 7.5% Al
and (c) Al-doped -Fe
2
O
3
with 13.1% Al.
To differentiate the possible difference of dimension and dispersion between
pure -Fe
2
O
3
and Al-doped -Fe
2
O
3
, synthesized Al-doped -Fe
2
O
3
with an Al/(Al + Fe)
Search WWH ::

Custom Search