Environmental Engineering Reference
In-Depth Information
1.0
B(62/36/2)
D(67/29/4)
C(72.5/21/6.8)
Bare nZVI
0.8
0.6
0.4
0.2
0.0
0
20
40
60
80
100
120
Time (min)
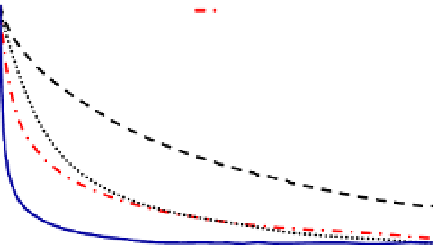
Figure 8.6
Results from nZVI sedimentation studies. B, C and D are samples of
polymer with different weight percents of polydimethylsiloxane (PDMS), polyethylene
glycol (PEG) and acrylic acid (AA). For example, polymer B (62/36/2) contains 62%
PDMS, 36% PEG and 2% AA.
To test the versatility of the APGCs in improving the dispersibility of
nanoparticles, silver nanoparticles (AgNP, 100nm, Aldrich) were also coated, and
sedimentation studies carried out. The results indicated that there is no change in
colloidal properties of AgNP when coated with the APGC. The sedimentation plot for
the coated AgNP was essentially the same as that of the bare particles (Figure 8.7). Ag
(molecular weight ~108 g mol
-1
) is almost two times heavier than Fe (molecular weight
~56 g mol
-1
), which may be one of the reasons why the AgNP sedimented out so fast.
Change in the APGC architecture might have enhanced the colloidal stability of the
particles but that was beyond the scope of the present research.
The surface coating on the nZVI was achieved by combining nZVI with APGC
and rotating them in a custom made end-over-end shaker for 72 h. The mixing allowed
the nanoparticles to disperse and the polymer to absorb onto the surface of the
nanoparticles. The mixing time of 72 h appeared a little long, and efforts were made to
shorten the mixing time. A 48-h mixing time gave similar results (Figure 8.8). The
authors infer that there is a possibility of shortening the mixing time and, thus, reduce
production cost of the coated nZVI. Further work is needed to optimize the mixing time.



Search WWH ::

Custom Search