Environmental Engineering Reference
In-Depth Information
reaction. The surface reaction usually involves several steps in the overall reaction
including the diffusion of a reactant to the surface, a chemical reaction on the surface,
and the diffusion of a product back into the solution. The rate-limiting step (i.e., the
slowest reaction step requiring the greatest activation energy) determines the overall
kinetics of a reaction. In general, a typical minimum value of the activation energy for
chemical-controlled reactions is ~29 kJ/mol (Brezonik, 1994). Activation energies of the
dechlorination reactions with bimetallic Pd/Fe nanoparticles and iron nanoparticles are
estimated to be 31.1 and 44.9 kJ/mol, respectively (Lien and Zhang, 2007). The decrease
of the activation energy indicated that the dechlorination by bimetallic Pd/Fe
nanoparticles is a catalytic reaction. Palladium on the iron surface serves as a catalyst.
Furthermore, the value of the activation energy also indicates that the surface-chemical
reaction rather than diffusion is the rate-limiting step for metal-mediated dechlorination.
Similar conclusion for the dechlorination of trichloroethylene and carbon tetrachloride
using ZVI was also proposed by Su and Puls (1999) and Scherer et al. (1997),
respectively.
0.3
0.3
0.2
0.2
0.1
0.1
0
0
Engineered bimetallic
Cu/Fe nanoparticles
Engineered bimetallic
Cu/Fe nanoparticles
In situ formed bimetallic
Cu/Fe nanoparticles
In situ formed bimetallic
Cu/Fe nanoparticles
Iron nanoparticles
Iron nanoparticles
$V'F
$V'F
$V
$V
'F
'F
Metal Type
Metal Type
Figure 7.14
Comparison of engineering and in situ formed bimetallic Cu/Fe
nanoparticles for the degradation of carbon tetrachloride.
Reductive dechlorination at metal surfaces involved either direct or indirect
reduction or both (Brewster, 1954; Li and Farrell, 2000). Direct reduction, such as
hydrogenolysis and -elimination in the transformation of trichloroethylene by iron, may
occur via formation of an organic chemisorption complex at the metal surface where
metal itself serves as a direct electron donor. Indirect reduction involves atomic
hydrogen and no direct electron transfer from metals to reactants occurring. Atomic
hydrogen is a very powerful reducing agent that reductively dechlorinates contaminants
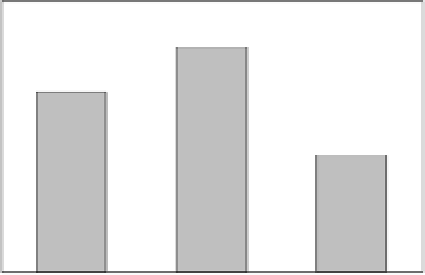
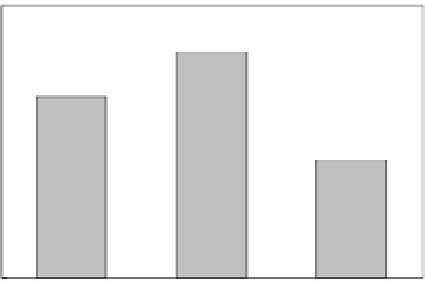









Search WWH ::

Custom Search