Environmental Engineering Reference
In-Depth Information
The overall E
o
for this reaction is +0.78 V at 25
o
C, indicating a strongly favorable
reaction from a thermodynamics perspective. The formation of Cu
2
O could be attributed
to the reaction between Fe(II) and Cu(II) in the aqueous solution (Maithreepala and
Doong, 2004):
2
+
2
+
+
(Eq. 7.14)
2Fe
+
2Cu
+
7H
O
→
Cu
O
+
2Fe(OH)
+
8H
2
2
3
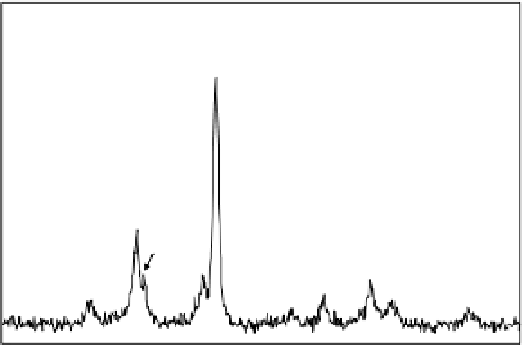
600
Fe
500
400
300
M
200
Cu
2
O
Cu
M
M
M
Fe
Cu
2
O
100
M
0
20
30
40
50
60
70
80
2
θ
Figure 7.12
XRD pattern of Cu(II)-treated iron nanoparticles. M represents magnetite
and/or maghemite.
As iron nanoparticles have the capability of the sequestration of heavy metals,
Table 7.5 summarizes the removal of selected metallic ions by iron nanoparticles. It can
be found that for metals with the standard reduction potential much more positive than
iron such as Cu(II), Ag(I), and Hg(II), the removal mechanism is mainly chemical
reduction (Li and Zhang, 2007). Metals with the standard reduction potential slightly
more positive than iron such as Ni(II) and Pb(II) can be immobilized at the surface of
iron nanoparticles by both adsorption and chemical reduction (Li and Zhang, 2007).
Clearly, this result indicates that in situ formation of bimetallic iron nanoparticles in the
site contaminated with these metallic ions is feasible.














Search WWH ::

Custom Search