Environmental Engineering Reference
In-Depth Information
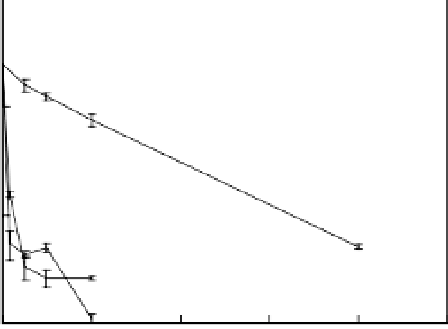
micro-sized iron particles at lower arsenic concentrations (Su and Puls, 2001). However,
a fast initial removal followed by a slow subsequent process was found in the case of
both Cu(II) and Pb(II). Disappearance of more than 93% of total Cu(II) and Pb(II)
occurred within 5 min. The first order reaction kinetics was simply not fitted with the
data. This result is consistent with studies conducted by Ponder et al. (2000). They
suggested a physical mechanism was involved in this type of removal processes. Overall,
the different removal behaviors among As(V), Cu(II) and Pb(II) suggest that the removal
of these heavy metals involves different mechanisms.
100
Cu(II)
Pb(II)
As(V)
10
1
0.1
0
1
2
3
4
5
Time (h)
Figure 7.11
Removal of heavy metals by iron nanoparticles.
The disappearance of metal ions in the aqueous solution was attributed to the
deposition at the iron nanoparticle surface confirmed by SEM-EDX analysis (data not
shown). The XRD pattern of the Cu(II)-treated iron nanoparticles taken after reacting
with 250 mg/L Cu(II) for 12 h is shown in Figure 7.12. Iron corrosion products
(magnetite and/or maghemite) were found at the surface. Two copper species, metallic
copper (Cu
0
) and cuprite (Cu
2
O) identified at the iron surface indicate the reduction of
Cu(II) by iron nanoparticles is involved. Because the standard reduction potential of
Cu
2+
/Cu
0
and Fe
2+
/Fe
0
couples is +0.34 and -0.44 V, respectively (Table 7.1), metallic
copper can be formed through the redox reaction:
Cu
2
+
+
Fe
0
→
Cu
0
+
Fe
2
+
(Eq. 7.13)










Search WWH ::

Custom Search