Environmental Engineering Reference
In-Depth Information
accumulation of reaction products (i.e., chloromethane and methane) was detected. The
formation of these reaction products indicates dichloromethane undergoes a reduction
reaction. The fast initial disappearance of dichloromethane implies that the removal of
dichloromethane involves the sorption occurring initially. Similar results have been
observed in many other surface-mediated processes where the rapid initial decrease of
reactants is attributed to the effect of sorption (Burris et al., 1998). This example
demonstrates the unique feature of bimetallic Cu/Al particles for the feasibility to
reductively degrade less-chlorinated organic compounds.
[AL-F-2.RAW] Al(7N)-Fresh
[AL-F-2.RAW] Al(7N)-Fresh
[AL-F-2.RAW] Al(7N)-Fresh
[AL-F-2.RAW] Al(7N)-Fresh
7000
7000
7000
7000
6000
6000
6000
6000
Al
Cu
Al(OH)
3
Al
Cu
Al(OH)
3
Al
Cu
Al(OH)
3
5000
5000
5000
5000
4000
4000
4000
4000
3000
3000
3000
3000
2000
2000
2000
2000
1000
1000
1000
1000
0
0
0
0
10
10
10
10
20
20
20
20
30
30
30
30
40
40
40
40
50
50
50
50
60
60
60
60
70
70
70
70
80
80
80
80
2-Theta(?
2-Theta(?
2-Theta(?
2-Theta(?
2θ
2θ
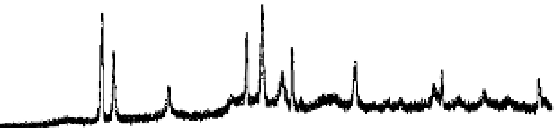
Figure 7.5
XRD pattern of fresh bimetallic Cu/Al particles.
2
2
CH2Cl2
CH3Cl
CH4
CH2Cl2
CH3Cl
CH4
CH
2
Cl
2
CH
3
Cl
CH
4
CH
2
Cl
2
CH
3
Cl
CH
4
1.5
1.5
1
1
0.5
0.5
0
0
0
0
100
100
200
200
300
300
Time (h)
Time (h)
Figure 7.6
Repetitive addition of dichloromethane in reaction with bimetallic Cu/Al
particles.






























































































































































































Search WWH ::

Custom Search