Environmental Engineering Reference
In-Depth Information
hydroxide/oxyhydroxides (Dixon and Weed, 1989). In the case of reacted bimetallic
Fe/Al particles, a group of small diffraction peaks appeared at 20.4°, 27.9°, 36.6°, and
40.7° is identified as the characteristic peaks of aluminum hydroxide (bayerite, Al(OH)
3
)
(Insert in Figure 7.2(c)).
7.2.1.2 Dechlorination of Carbon Tetrachloride
The degradation of carbon tetrachloride by bimetallic Fe/Al particles was
evaluated in a batch experiment, during which a dose of 20-40 μmol carbon tetrachloride
was repeatedly spiked into a 100-mL solution containing 1 g bimetallic Fe/Al particles.
The total operation time was about 320 h. The added carbon tetrachloride was nearly
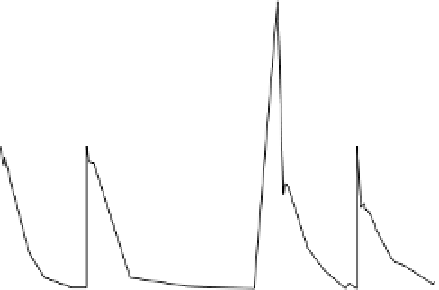
completely degraded within about 60 h for each cycle (Figure 7.3). Small amounts of
chlorinated intermediates (chloroform and dichloromethane) were produced, but no
significant accumulation was observed until 40 μmol of carbon tetrachloride was added.
The accumulated amount of dichloromethane was about 10% of the total addition of
carbon tetrachloride in the four- cycle experiments.
45
45
40
40
CCl4
CHCl3
CH2Cl2
CCl4
CHCl3
CH2Cl2
CCl
4
CHCl
3
CH
2
Cl
2
CCl
4
CHCl
3
CH
2
Cl
2
35
35
30
30
25
25
20
20
15
15
10
10
5
5
0
0
0
0
50
50
100
100
150
150
200
200
250
250
300
300
Time (h)
Time (h)
Figure 7.3
Repetitive addition of carbon tetrachloride in reaction with bimetallic Fe/Al.
The observed rate constant of contaminants can be estimated using Eq. 7.4:
dC
=
−
k
C
(Eq. 7.4)
obs
dt
where C is the concentration of carbon tetrachloride (mg/L);
k
obs
is the observed rate
























































































Search WWH ::

Custom Search