Environmental Engineering Reference
In-Depth Information
1998).
In the following section, research on polymeric nanoparticles is presented
therein to illustrate their potential in heavy metal removal. Typical examples are also
included in Table 6.3 for easy reference.
(a)
(b)
(c)
(d)
Figure 6.1
General morphology of polymeric nanoparticles: (a) homogeneous sphere;
(b) core/shell sphere; (c) microgel; and (d) dendronized sphere.
6.3.2 Recent Research Activities
6.3.2.1 Poly(N-Isopropylacrylamide)-Based Nanoparticles
Many types of polymers have been synthesized into nanoparticles with the
aids of currently available polymerization techniques and toolkits. One of the first
environmental applications of polymeric nanoparticles was investigated by Snowden
and Vincent (1993), who explored the applicability of using poly(N-
isopropylacrylamide) P(NIPAAm) nanoparticles (referred as “colloidal microgels” by
therein) to remove heavy metals such as Pb(II) and Cd(II). Poly(acrylamide)-based
polymers have long been used as polymeric flocculant in the clarifier unit commonly
found in conventional water/wastewater treatments. However the preparation of
poly(acrylamide) nanoparticles was plagued with synthetic difficulty, due to the high
polarity of the precursor monomer. Therefore the homologues of poly(acrylamide)
with reduced polarity (i.e. higher hydrophobicity) was explored for alternative
starting materials. P(NIPAAm) was chosen due to the simplicity of using the methods
for production of nanoparticles. The binding sites for metal ions were derived from
the thermal initiator molecules used. Hence, the removal capacity for both heavy
metal ions was limited as the ratio of the initiator to N-isopropylacrylamide was kept
small in all the synthesis therein. For instance, the amount of lead ions adsorbed was
only 0.4 mmol/g at solution pH = 6.0. Polymers made from NIPAAm are known to
exhibit thermosensitive properties (Saunders and Vincent, 1999). Snowden and
Vincent (1993)
observed that the solutes retained by the P(NIPAAm) nanoparticles
were released when the temperature rose to 50 °C.
This shortcoming in adsorption capacity was later overcome by Morris et al
.
(1997) by synthesizing P(NIPAAm) together with a second comonomer, acrylic acid.
The carboxylic acid groups (-COOH) of the synthesized polymer dissociates
completely and becomes negatively charged (-COO
-
) at pH above 4. It was reported
that an improved performance (e.g., a specific adsorption capacity of 2.4 mmol/g was
achieved for Pb(II) at pH = 8.0) was attributed to the favorable Coulombic attraction







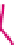


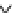
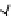
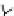






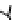
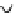





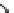
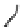
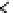
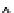
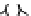
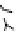


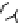
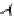
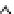

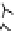

























Search WWH ::

Custom Search