Environmental Engineering Reference
In-Depth Information
50
40
30
Influent
20
Effluent
10
0
0
10000
20000
30000
40000
50000
Bed volumes
Figure 5.11
Arsenic removal from groundwater using the TiO
2
adsorbent. Continuous
filtration at a flow rate of 1 L/min; empty bed contact time (EBCT) = 3 min.
The results show that the effluent arsenic concentration is below the detection
limit of 1 μg/L in the first 25,000 bed volumes of filtered water. The effluent arsenic
concentration increased gradually after 25,000 bed volumes of water were filtered.
Approximately 45,000 bed volumes of water had been treated by the TiO
2
filter when
the effluent arsenic increased to 10 μg/L. The volume of water treated is about 45,000
liter per 1 liter of adsorbent or 60,000 liter per 1 kg of adsorbent.
5.3.5 Organic Arsenic Removal with Nanocrystalline TiO
2
Compared to a large body of literature on removal of inorganic arsenic from
water, only a few studies have been conducted to investigate the treatment of organic
arsenic in water (Jing et al., 2005). The adsorption of MMA and DMA on
nanocrystalline TiO
2
has been studied at a constant ionic strength of 0.04 M as KNO
3
.
The adsorption edge is shown in Figure 5.12. The characteristics of MMA and DMA
adsorption on TiO
2
are similar to inorganic As(V) (Figure 5.6), which has a high
adsorption percentage at low pH and decreases to nearly zero within a narrow pH range.
MMA adsorption reached almost 100% when the pH was less than 7.5, while a
maximum adsorption of 65% was achieved for DMA at pH around 5.5.
EXAFS spectroscopy has been employed to determine the arsenic local
coordination environment of MMA and DMA in the adsorbed phases. The k
3
weighted
As K-edge EXAFS spectra are shown in Figure 5.13A for MMA and DMA on the TiO
2
surface. The corresponding radial structure functions (RSF) are depicted in Figure 5.13B
as Fourier transform (FT) vs. radial distance.









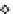


Search WWH ::

Custom Search