Environmental Engineering Reference
In-Depth Information
As = 0
As(V) = 50 μg/L
As(III) = 50 μg/L
As(V) = 100 μg/L
As(III) = 100 μg/L
30
20
10
pH
0
3
4
5
6
7
8
9
-10
-20
-30
-40
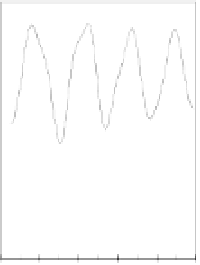
Figure 5.7
Zeta potential of 10 mg/L TiO
2
as a function of pH and total As
concentration in 0.04 M NaCl solution.
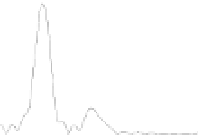
The extended X-ray absorption fine structure (EXAFS) spectroscopy was
developed as a quantitative, short-range structural probe in the 1970's following the
pioneering work of Sayers, Stern, and Lytle (Sayers et al., 1971). Among the most
important applications of EXAFS in the environmental field is the study of As surface
complexation at liquid-mineral interfaces. Figure 5.8 shows the As K-edge EXAFS
spectra, and modeling results are summarized in Table 5.1.
(A)
(B)
As-O
As(V)
As-Ti
As(V)
As(III)
As(III)
3
5
7
9
11
13
0
1
2
3
4
5
6
k
(Å
-1
)
R (Å)
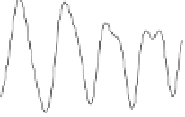
Figure 5.8
(A) The k
3
weighted observed (dotted line) and model calculated (solid line)
As K-edge EXAFS spectra and (B) Fourier transform magnitude resulting in a radial
distance structure for the As(V) and As(III) adsorption on TiO
2
at pH 7. The peak
positions are uncorrected for phase shift.




















































Search WWH ::

Custom Search