Environmental Engineering Reference
In-Depth Information
agreement with an average value of 5.9 reported in the literature for the PZC of TiO
2
(anatase) (Kosmulski, 2002).
0.2
0.1M
0.01 M
0.1
0
2
4
6
8
10
pH
-0.1
-0.2
-0.3
Figure 5.2
Surface charge of TiO
2
as a function of pH in 0.1 M and 0.01 M KNO
3
solution.
The zeta potential () has been measured for 0.01 g/l TiO
2
solutions with 0.1,
0.04, 0.01, and 0.001 M KNO
3
in the pH range of 3 to 9. The experimental potential
values as a function of pH are shown in Figure 5.3. The isoelectric point (IEP) can be
determined from the intersection point of the potential curves, which occurs at pH of
5.8, identical to the PZC.
40
30
0.001 M
0.01 M
0.04 M
0.1 M
20
10
0
pH
2
4
6
8
10
-10
-20
-30
-40
Figure 5.3
Experimental zeta potential values as a function of pH in 0.001, 0.01, 0.04,
and 0.1 M KNO
3
solutions.







































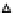



Search WWH ::

Custom Search