Environmental Engineering Reference
In-Depth Information
Cl
Cl
Cl
Cl
Cl
Cl
Cl
2,2',5,5'-tetrachlorobiphenyl
Cl
Cl
Cl
2,2',5-trichlorobiphenyl
2,3',5-trichlorobiphenyl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
3,3'-dichlorobiphenyl
2,2'-dichlorobiphenyl
2,3'-dichlorobiphenyl
2,5-dichlorobiphenyl
Cl
Cl
3-chlorobiphenyl
2-chlorobiphenyl
biphenyl
Figure 4.2
Possible stepwise degradation of 2,2',5,5'-tetrachlorobiphenyl (BZ# 52) by
microscale ZVI (adapted from Yak et al., 2000).
4.5
Application of Other Nanoscale Metallic Particles in Chlorinated
Organic Compound Degradation
In a recent study (Nutt et al., 2005) researchers investigated enhanced catalytic
performance for TCE dechlorination by bimetallic palladium nanoparticles supported on
gold nanoparticles (Au NPs) with different Pd loadings. The bimetallic palladium-gold
nanoparticles were synthesized by controlling Pd loading to the Au sol with gold
nanoparticle core diameter of 20 nm. The highest TCE dechlorination rate of 943
L/g
Pd
/min was achieved by the bimetallic NPs with Au NPs partially covered by 1.9 wt.
% of Pd metal content. The TCE dechlorination rate was much faster than that of using
palladium nanoparticles only (62.0 L/g
Pd
/min).
For the purpose of cost reduction the same research group (Nutt et al., 2006)
investigated similar Pd-on-Au bimetallic nanoparticles with gold nanoparticle core
diameter of 4 nm and with different Pd loadings in a following study. A much higher
TCE dechlorination rate of 1956 L/g
Pd
/min than that of previous study was achieved



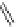
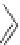


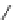


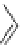


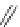
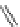
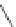

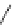

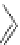
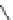




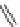
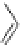


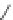

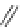
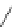
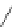

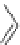
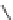
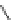

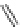


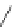


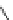
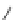


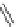

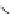


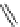
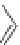
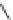
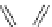

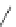
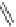


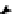












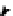



























































Search WWH ::

Custom Search