Civil Engineering Reference
In-Depth Information
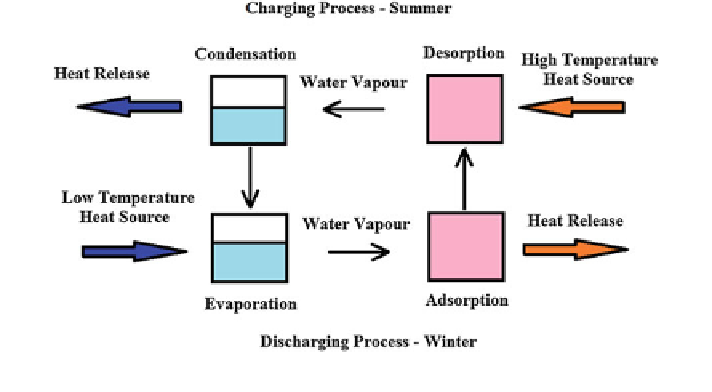
Fig. 9
Operation principle of closed adsorption system
In the discharging cycle, based on the energy demand and the thermal load
conditions prevailing in the evaporator, the stored water is evaporated, and the
vapour is then mixed with the dry silicagel (adsorbent) in the adsorber store. By
this, the silicagel captures the water vapour through adsorption phenomenon and
eventually releases back the stored useful heat energy. Roughly, the storage
density of this system was accounted to be 150 kWh/m
3
of silicagel.
4.9.2 Open Adsorption Energy Storage System
The open adsorption energy storage system also offers an attractive way for storing
and releasing the heat energy upon energy demand requirements in buildings. The
Institute of Thermodynamics and Thermal Engineering (ITW) in the University of
Stuttgart (Germany) has proposed an open adsorption storage named as ITW
Monosorp (N'Tsoukpoe et al.
2009
). This system also utilizes the high-grade solar
energy as the prime heat source for desorption process to take place in the adsorber
store.
In principle, during the regeneration cycle (especially in summer), the solar heat
energy being trapped using the evacuated tube solar collectors and which is
available at a temperature of 180-190 C is fed as the heat input to the incoming
ambient air through a dedicated heat exchange as shown in Fig.
10
.
The heated air is then passed over the zeolite 4A material which is filled inside
the adsorber store. This enables desorption of zeolite 4A to take place by virtue of
vaporizing the water content from the reactive compound. The return warm air is
exhausted to the ambient after preheating the fresh incoming air.
During the discharging period (especially in winter), the wet/moist air from the
indoor spaces is allowed to flow over the adsorbent store, wherein the water