Civil Engineering Reference
In-Depth Information
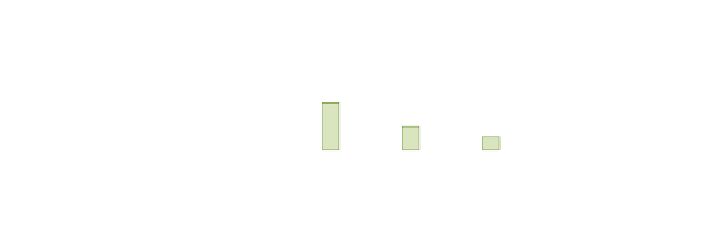
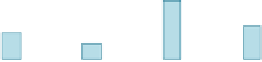
Fig. 2 Personal 48-h
exposure to CO (PE) for non-
smokers and smokers, and
ambient concentrations of CO
in five European cities
(Georgoulis et al.
2002
). Bars
indicate standard errors
5.0
4.0
3.0
2.0
1.0
0.0
Athens,
Greece
Basle,
Switzerland
Helsinki,
Finland
Milan, Italy
Prague,
Czech
Republic
PE, non-smokers
PE, smokers
outdoor
ranged between 0.01 and 10 mg/m
3
, with maximum values of 100 mg/m
3
in
traffic-affected buildings (Kolarik 2012). Similar values were found in the resi-
dences in the EXPOLIS study (Georgoulis et al.
2002
). Mean personal exposure at
homes for non-smokers and for smokers, and outdoor concentrations of CO were
reported (see Fig.
2
). Exposure and ambient concentrations differ significantly
between cities, due to differences in size and socio-economic characteristics. The
higher values were reported in Athens and Milan, and the lowest in Helsinki.
Generally, ETS increased the short-term exposure to CO, but it was also reported
that cooking with gas leads to higher CO concentrations. Ambient concentrations
had also a large influence on personal exposure.
Inhalation is the only exposure route to CO for humans. CO rapidly enters
blood, brain, hearth and muscles, causing severe short- and long-term effects. CO
is eliminated from the body mainly by exhalation (ATSDR
2013b
). Symptoms
from acute exposure include headache, weakness or lethargy, dizziness, nausea or
vomiting, and difficulties with memory or confusion (Weaver et al.
2002
), being
more vulnerable people suffering respiratory or cardiovascular diseases. In order to
address short-term CO exposure, WHO considered 15 min, 1 h, and 8 h guideline
values, shown in Table
2
. Long-term exposure can cause cardiovascular diseases
(WHO
2010
) and is addressed by the 24-h guideline value.
2.3 Nitrogen Dioxide
In atmospheric chemistry, the term NO
X
means the total concentration of nitric
oxide (NO) and nitrogen dioxide (NO
2
). They are produced in combustion
processes, when nitrogen and oxygen react, especially at high temperatures.
Typically, more than 90 % of the nitrogen oxides are emitted as NO, which in
ambient air is oxidised with ozone to form NO
2
(Derwent and Hertel
1999
). NO
X
are tropospheric ozone precursors and react to produce acid rain, and, hence, they
are limited under Directive
2008
/50/EC. WHO guidelines for IAQ include NO
2
,
but not NO because there is no sufficient evidence for it (WHO
2010
).