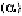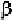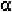Biology Reference
In-Depth Information
Table 2-1. Amino acid sequences of sperm whale myoglobin and
and
subunits of human
hemoglobin (modified from Perutz, 1962).
|-----A------| |--------B- --------| |--C--|
Mb
VAGEWSEILKXWAKVQALVAGHGKLTLIRLFKSHPETLEKFDRFKHLK
Hb
VLSPADKTNVKAAWGKVGAHAGEYGAEALERMFLSFPTTKTYFPHF-DLS
Hb
VHLTPEEKSAVTALWGKVN--VDEVGGEALGRLLWYPWTQRFFESFGDLS
|--D--| |--------E---------| |---F---|
Mb
TEAEMKASEDLKVHGIEVDTALGAILKKKGHHELEALPKAESHAKLFKI
Hb
H-----GSAQVKGHGKKVADALTNAVAHVDDMPNALSALSDLHAHKLRV
Hb
TPDAVMGNPKVKAHGKKVLGAFSDGLAHLNDLKGTFATLSQLHCDKLHV
|-------G--------| |----------H----------|
Mb
PIKYXEHLSXAVIHVRATKHDDEFGAPADGAMDKALELFRKDIAAKYKELGYGE
Hb
DPVNFKLLSHCLLVTLAAHLPAEFTPAVHASLDKFLASVSTVLTSKWR
Hb
DPENFRLLGNVLVCVLAHHFGKEFTPPVQAAYQKWAGVANALAHKWH
Subsequently, the myoglobin sequence has been revised (Edmundson, 1965)
and realigned (Eck and Dayhoff, 1966):
VLSEGEWQLVLHVWAKVEADVAGHGQDILIRLFKSHPETLEKFDRFKHLKTEAEMKASED
LKKHGVTVLTALGAILKKKGHHEAELKPLAQSHATKHKIPIKYLEFISEAIIHVLHSRHP
GNFGADAQGAMNKALELRFKDIAAKYKLEGYQG
These two proteins have a high content of -helices, the locations of which
are given in Table 2-1. Each chain is associated with one heme (Fig. 2-1).
Heme can be physically dissociated from these proteins, and the -helical
contents are drastically reduced. Their three-dimensional structures are
similar. However, the association of the two -subunits and two -subunits
of the hemoglobin molecule is complicated, plays a vital role of the function
of the entire protein, and has been extensively studied by Perutz (1998). A
schematic representation of the three dimensional arrangements of these
subunits in hemoglobin can be found in Perutz (1962). More detailed three