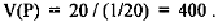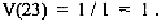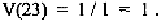Biology Reference
In-Depth Information
7
My friend, Dr. Kabat, and I were staring at such alignments on long strips of
yellow papers placed on a large conference table, and realized that we had to
introduce some quantitative measure. Biochemists have long realized that
for most of the proteins, there are only twenty different ammo acid residues
linked linearly. We reasoned that if the lengths of the variable regions of
light chains were so similar, their segments responsible for the binding of
antigens would consist of different amino acid residues from sequence to
sequence. This situation is in complete contrast to proteins with one specific
function. For example, cytochrome c's from different species have different
sequences. However, since they all serve the same function of electron
transport, their active sites should consist of similar or identical amino acid
residues.
Our analysis had to be simple. Otherwise, immunologists or molecular
biologists would not appreciate our findings. After many attempts, we
settled on the following ratio as a function of position, and named it
variability (Wu and Kabat, 1970):
where V is the variability, a function of position P. For a set of aligned
amino acid sequences, the numerator N is the number of different amino
acid residues found at that position. The denominator D is the frequency of
the most common amino acid residue at that position. For example, if at
position 23, Cys is found in all sequences, we have N = 1, and D =1. Thus,
Therefore, variability is equal to one for an invariant position. On the other
hand, the theoretical maximum of V is for a position P where all twenty
amino acid residues are found in different sequences, and they occur at the
same frequency, i.e. N = 20, and D = 1/20. Then, for that position P, we
have: