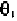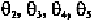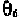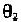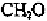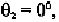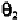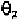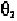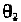Biology Reference
In-Depth Information
182
The negative values of tolerance in Figs. 7-3 and 7-4 suggest that the van
der Waal radii of various atoms listed in Table 7-1 should be reduced
somewhat, especially that for H atom. However, roughly, we have possible
values of
for pyrimidine and purine in the ranges:
pyrimidine:
95
0
to 130
0
, and 230
0
to 315
0
;
purine:
40
0
to 160
0
, and 220
0
to 320
0
.
The value of 100
0
corresponds to the
syn
configuration suitable for the
Hoogsteen pairing (Hoogsteen, 1963), and that of 280
0
to the
anti
configuration suitable for the Watson-Crick pairing (Watson and Crick,
1953). Indeed, especially for purine bases, there is a fairly large amount of
allowable rotation around the glycosidic bond.
FIVE DEGREES OF FREEDOM OF THE BACKBONE
Rotations around the five single bonds (Metzler, 1977) between two
successive phosphorous atoms as shown in Fig. 7-1 have designations
overlapping with those for the polypeptide backbone. To avoid such
confusion, earlier in 1968, they were designated as and (Wu,
1968). The C
4
'-C
3
' bond will be considered separately due to the
restrictions imposed by the ribose ring structure.
The angle is defined in Fig. 7-5, for the rotation around the C
4
'-C
5
' bond.
The C
4
' atom is positioned at the origin, and the C
4
'-C
5
' bond along the
negative z-axis. The group of the sugar lies below the x,y-plane, and
is held stationary with the O atom in the x,z-plane. The ribose ring is then
rotated around the z-axis in the positive direction, with the right-hand rule.
At all solid bonds in Fig. 7-5 are in the x,z-plane. The angle is the
angle between the x,z-plane and the plane formed by the C
3
'-C
4
' and
C
4
'-C
5
' bonds. Again for calculation of tolerance only those atoms
shown in Fig. 7-5 are included. Tolerance as a function of is plotted in
Fig. 7-6. The least hindered values of
are around 120
0
and 240
0
. Most of
the values of
seem to be allowed, except possible between 130
0
to 230
0
.