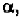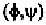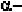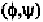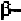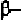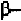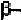Biology Reference
In-Depth Information
142
SECONDARY STRUCTURES OF POLYPEPTIDES
If a linear polypeptide has the same angles for every amino acid
residue, it will fold up into a periodic structure, commonly referred to as the
secondary structure. The most well known one is the right-handed helix
originally predicted by Pauling
et al.
(1951) theoretically, and verified
experimentally soon afterwards. It consists of 3.6 amino acid residues per
turn, and is stabilized by the presence of hydrogen-bonds between the
backbone N-atoms and the carbonyl O-atoms. The ring structure thus
formed contains 13 atoms. It is therefore sometimes referred to as a 3.6
13
helix. In order to form these hydrogen-bonds, the angles are roughly
in the range of (-48
0
,-57
0
) to (-67
0
,-44
0
) in the new convention, so that the
helix can have some flexibility (Dickerson and Geis, 1969). In the case
of myoglobin and hemoglobin (Chapter 2), nearly 80% of the protein is in
the helical configuration. However, there is some debate as to whether these
helices are i.e. 3.6
13
, or 3
10
which is slightly different with three amino
acid residues per turn and the ring structure formed by hydrogen-bond
having 10 atoms. The 3
10
-helices have different
angles (Dickerson
and Geis, 1969).
Atomic models can be generated using these values, the translation
and rotation of coordinates as discussed in the Appendix, and numerical
computations with programming. These are very interesting studies but
beyond the score of this topic. However, using repeated peptide units
represented in Fig. 5-3, it is possible to get an idea of the three dimensional
structure of the -helix.
Another important secondary structure is the strand. It is nearly straight
with two amino acid residues per “turn”. When aligned, several of them can
form either anti-parallel or parallel pleated sheet with hydrogen-bonds.
Several sheets may be stacked together for stability as in the case of
antibody molecules (Chapter 1). For anti-parallel strands, their
values are around (-139
0
, +135
0
); and for parallel strands, around
(-123
0
,+116
0
). Similarly, these structures can be visualized by using the
paper model illustrated in Fig. 5-3.
There are many other secondary or periodic structures of polypeptides,
discussed by Dickerson and Geis (1969). Most of them are right-handed,
but some are left-handed.