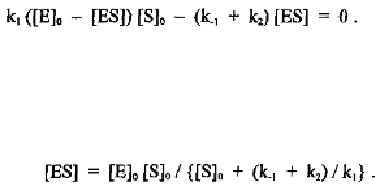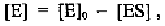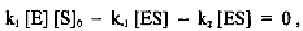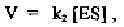Biology Reference
In-Depth Information
95
In most
in vitro
experimental measurements, [S]
0
is of the order of 10
-6
M or
larger while [E]
0
is of the order of 10
-8
M. However, in metabolic pathways
involving a number of consecutive enzymes, this assumption may not be
valid. With this assumption, the rate equation involving d[S]/dt must be
ignored.
As a result, we have the following set of algebraic equations:
and
where V is the velocity of the reaction and is equal to d[P]/dt. We can thus
express [E] in terms of [E]
0
and [ES], and express [ES] in terms of [E]
0
and
[S]
0
, i.e.
which can be substituted into the equation with the kinetic constants to give:
From this equation, [ES] can be expressed in terms of the three kinetic
constants and the initial concentrations of the enzyme and substrate:
Finally, we can calculate the velocity of the simple enzyme kinetic reaction: