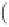Geoscience Reference
In-Depth Information
▲
Revil and Skold (2011) (NaCl, 0.1 S m
-1
) clean sand
Vinegar and Waxman (1984), Sandstone 0.1 M NaCl
Revil et al. (2013), Saprolite 0.1 M NaCl
Model
σ
ʺ=−
b
CEC
M
Slater and Glaser (2003) (NaCl, 0.1 S m
-1
) sandy sediments
Lesmes and Frye (2001) (NaCl, 0.1 S m
-1
) berea sandstone
+
Revil et al. (2013) (NaCl, 0.1 S m
-1
) saprolites
Weller et al. (2011) (NaCl, 0.1 S m
-1
) sandstones
Revil et al. (2013) (NaCl, 0.1 S m
-1
) clean sandstones
Revil et al. (2013) (NaCl, 0.1 S m
-1
) clayey sandstones, mudstone
Börner (1992) (NaCl, 0.1 S m
-1
, sandstone)
Koch et al. (2011) (NaCl, 0.04-0.06 S m
-1
, clean sands)
10
-3
10
-1
10
-2
10
-4
+
+
+
+
+
+
+
10
-3
+
10
-5
10
2
10
4
Cation exchange capacity, CEC (C kg
-1
)
10
3
10
-4
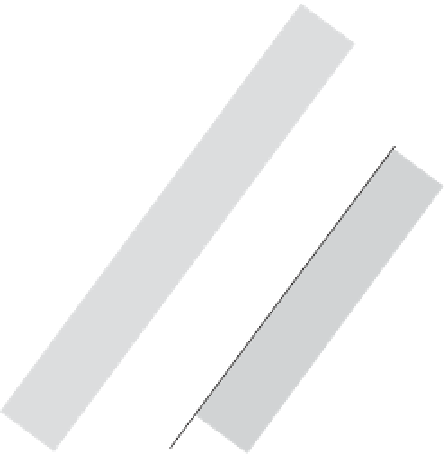
Figure 1.15
Influence of the cation exchange capacity (CEC)
upon the quadrature conductivity of clayey materials. The trend
is determined for the clayey materials from the model developed
by Revil (2012, 2013) at 0.1 mol l
−
1
NaCl (about 1 S m
−
1
). The
measurements are from Vinegar and Waxman (1984) (shaly
sands) and Revil et al. (2013) (saprolites). Note that the slope of
this trend is salinity dependent.
▲
10
-5
10
5
1
10
100
1,000
10,000
S
sp
(m
2
kg
-1
)
Speciic surface area,
Figure 1.14
Influence of the specific surface area
S
Sp
upon the
quadrature conductivity, which characterizes charge
accumulation (polarization) at low frequencies. The trend
determined for the clean sands and the clayey materials are from
the model developed by Revil (2012) at 0.1 S m
−
1
NaCl. The
measurements are reported at 10 Hz. Data from Revil and Skold
(2011), Koch et al. (2011), Slater and Glaser (2003), Lesmes and
Frye (2001), Revil et al. (2013), and Börner (1992).
of quadrature,
σ
s
. With this
definition, the complex conductivity of a partially satu-
rated porous siliciclastic sediment can be written as
σ
, to surface conductivity,
σ
∗
=
1
F
σ
w
1 +Du 1
−
i
R
1 93
of the CEC. The data are corrected for the dependence
of the partition coefficient
f
with the salinity using the
approach developed by Revil and Skold (2011). These
data exhibit two distinct trends indicating that the mobil-
ity of the counterions in the Stern layer of silica is equal to
the mobility of the same ions in the bulk pore water,
while the mobility of the counterions at the surface of
clays is much smaller than in the bulk pore water. For
clayey materials, it is also clear that the surface conduc-
tivity can be directly related to the quadrature conductiv-
ity as discussed by Revil (2013a, b).
The following dimensionless number can be defined as
Du =
F
σ
S
σ
1 94
w
As briefly discussed by Revil and Skold (2011) and
Revil (2012, 2013a), the ratio
R
can be related to the
partition coefficient
f
. In the present case, we obtain
S
β
+
f
R
=
1 95
S
+
f
β
+
1
−
f
+
β
S
+
f
β
+
1
β
R
clay
≈
1 96
−
f
R
≡−
σ
σ
S
≥
0, which corresponds therefore to the ratio