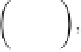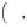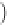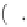Geoscience Reference
In-Depth Information
There are three distinct microscopic electric potentials
in the inner part of the electrical layer. We note
C
=1+
C
f
Na
+
K
3
φ
β
k
b
T
e
exp
−
1 65
φ
0
as the
mean potential on the surface of the mineral (Figure 1.1).
The potential
φ
d
is the
potential at the OHP (Figure 1.1). These potentials are
related to each other by a classical capacitance model
(Hunter, 1981):
φ
β
is located at the
β
-plane, and
where
e
is the elementary charge (in C),
T
is the temper-
ature (in degree K),
k
b
is the Boltzmann constant,
0
i
Γ
is
0
3
(in sites
per nm
2
) are the total surface site densities of the three
type of sites introduced earlier (aluminol, silanol, and
>Al
0
1
,
0
2
,
the surface site density of site i, and
Γ
Γ
Γ
φ
0
−
φ
β
=
Q
0
C
1
Si< groups, respectively). The parameters
C
i
where i =Na
+
,H
+
-
O
-
1 68
are the ionic concentrations (in
mol l
−
1
), and
φ
β
are the electrical potentials at
the mineral surface (o-plane) and at the
φ
0
and
Q
S
C
2
φ
β
−
φ
d
=
−
1 69
-plane, respec-
tively (Figure 1.1). The resulting mineral surface charge
density,
Q
0
, and the surface charge density in the Stern
layer,
Q
β
(in Cm
−
2
), are found by summing the surface
site densities of charged surface groups (see Leroy &
Revil, 2004).
In the case of smectite and illite, the surface site densi-
ties are located mainly on the basal plane {001}
(Tournassat et al., 2004). We use the TLM developed
by Leroy et al. (2007) to determine the distribution of
the counterions at the mineral/water interface of 2:1 clay
minerals. In the pH range 6
β
where
C
1
and
C
2
(in F m
−
2
) are the (constant) integral
capacities of the inner and outer parts of the Stern layer,
respectively (Table 1.2). The parameter
Q
S
represents the
surface charge density in the diffuse layer. The global
electroneutrality equation for the mineral/water inter-
face is
Q
0
+
Q
β
+
Q
S
=0
1 70
We calculate the potential
φ
d
by using Equations
8, the influence of the
hydroxyl surface sites upon the distribution of the coun-
terions at the mineral/water interface can be neglected
because the charge density induced by edge sites is small
relative to that due to permanent excess of negative
charge associated with the isomorphic substitutions
inside the crystalline network of the smectite
(Tournassat et al., 2004). We therefore consider only
these sites in the model denoted as the
-
(1.68)
(1.70) and the procedure reported by Leroy and
Revil (2004) and Leroy et al. (2007) (the surface charge
densities are expressed as a function of the corresponding
surface site densities). We use the values of the equilib-
rium constants
K
i
and of the capacities
C
1
and
C
2
reported
in Table 1.2. The system of equations was solved inside
twoMATLAB routines, one for kaolinite and one for illite
and smectite. The counterions are both located in the
Stern and in the diffuse layer. For all clay minerals, the
fraction of counterions located in the Stern layer is
defined by Equations (1.50)
-
(see
Figure 1.8). The adsorption of sodium is described by
“
X-sites
”
-
(1.52) like for the silica
>X
−
+Na
+
,
K
4
>XNa
1 66
surface.
C
f
Na
+
K
4
e
φ
β
k
b
T
0
XNa
=
0
X
Γ
Γ
exp
−
1 67
Table 1.2
Optimized double layer parameters for the three main
types of clay minerals (at 25 C).
The mineral surface charge density
Q
0
(in Cm
−
2
)of
smectite associated with these sites is considered equal
to the ratio between the CEC of smectite (1 meq g
−
1
)
and its specific surface area (800 m
2
g
−
1
), which gives a
value equal to 0.75 charge nm
−
2
(for illite, a similar anal-
ysis yields 1.25 charges nm
−
2
). These values allow the cal-
culation of the surface site densities
Γ
Parameters
Kaolinite
Illite
Smectite
10
−
10
K
1
(at 25 C)
—
—
8 × 10
−
6
K
2
(at 25 C)
—
—
5 × 10
−
2
K
3
(at 25 C)
—
—
0.8
†
0.8
†
K
4
(at 25 C)
—
C
1
(F m
−
2
)
1
†
1
†
1.58
XNa
knowing
the expressions of the mineral surface charge density
Q
0
(in Cm
−
2
) as a function of the surface site densities (see
Leroy et al., 2007).
X
and
Γ
C
2
(F m
−
2
)
0.2
0.2
†
0.2
†
From Leroy and Revil (2004).
†
From Leroy et al. (2007).